A rare case of adrenal gland abscess due to anaerobes detected by metagenomic next-generation sequencing
Introduction
Adrenal gland abscess is rare in adults but less uncommon in newborns (1). It usually occurs in patients with conditions such as immunosuppression, HIV infection, or recent surgical procedures. Adrenal gland infection can develop through either the dissemination of a bloodstream infection or previous extravasated blood in the adrenal gland, which is more common. Staphylococcus aureus and Escherichia coli are the most common pathogens according to the literature (2), and Streptococcus, Salmonella, Bacteroides, Echovirus, Herpes simplex virus, Cytomegalovirus, Nocardia, Tuberculosis, fungi and parasites have also been reported (3-6). Here, we report a case of adrenal gland abscess caused by multiple pathogens, predominantly mixed anaerobes, identified via metagenomic next-generation sequencing (mNGS) and culturing of Escherichia coli, Proteus mirabilis and Actinomyces turicensis. Next-generation sequencing technology identified specific nucleotides of the microbes in the samples and compared them to a DNA library through DNA fragmentation, end-repair, adapter-ligation and PCR amplification to identify possible pathogens in sterile areas (7-9). This technique is marked by its rapid diagnostic value and the advantage of simultaneously identifying multiple pathogens.
Case presentation
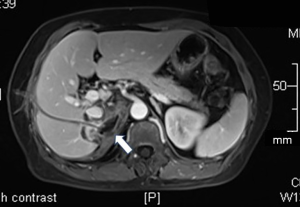
A 50-year-old previously healthy woman complained of right back pain and a low-grade fever of a 10-day duration. A previous abdominal CT scan showed a 7 cm-sized mass with a well-defined, thick wall, and laboratory testing showed leukocytosis (WBC count of 13,900 cells/mL) and an elevated C-reaction protein (CRP) level of over 90 mg/L. No improvement in symptoms occurred after the woman was prescribed ceftizoxime and metronidazole. For the further evaluation of malignancy, she underwent PET/CT scans, which revealed a hypodense lesion in the right suprarenal region with elevated SUV (6.7 for maximum). (Figures 1A,B) For further diagnosis, she was referred to the Department of Infectious Diseases, Zhongshan Hospital, Fudan University on 16th May, 2018.
Continuous worsening back pain without fever was found at admission, and the physical examination showed percussion pain on her right back. Admission labs showed leukocytosis (WBC count of 12,130 cells/mL) with elevated CRP (174.4 mg/L) and ESR (116 mm/Hg). Procalcitonin and tumor biomarkers were normal, and T-SPOT.TB was negative. Enhanced upper abdominal magnetic resonance imaging (MRI) showed right adrenal gland mass (7.6×6.5 cm) hypointensity in the T1-weighted images and hyperintensity in the T2-weighted images; the mass had a thick wall with an inhomogeneous signal inside (Figure 1C,D,E).
CT-guided percutaneous puncture catheter drainage was performed on the second day, and approximately 300 mL of foul-smelling thick pus was drained for routine examination, smear, culture (including aerobic, anaerobic and fungal cultures) and mNGS detection. The rhythm of the adrenocorticotropic hormone and cortisol secretion, the renin-angiotensin-aldosterone system (RAAS) hormone level and the secretion of methoxy epinephrine and methoxy norepinephrine were all assessed and were within the normal ranges. Exfoliated tumor cells were not found in the pus. The patient was preliminarily diagnosed with adrenal abscess after finding both gram-positive cocci and gram-negative bacilli in the pus. Therefore, we immediately initiated ertapenem therapy at 1 g every 24 hours intravenously. A transient fever (39.0 °C) with chill was observed 3 hours after drainage, and then, peripheral blood was drawn for culture and mNGS detection. The pus culture revealed only Escherichia coli (d2), Proteus mirabilis (d2) and Actinomyces turicensis (d5). By contrast, pus mNGS identified multiple pathogens (86% anaerobes) with high reads (including Peptostreptococcus 27.9%, Porphyromonas 19.2%, Filifactor 14.1%, Pyramidobacter 11.1%, Prevotella 9.9%, Anaeroglobus 1.1%, Bacteroides 1.1%, Slackia 0.9%, and Fusobacterium 0.7%) and other microorganisms (including Escherichia 0.1%, Proteus 0.6%, Mycoplasma 1.6% and so on) (Figure 2A). The blood culture was negative, whereas peripheral blood NGS detected multiple pathogens, including 74.6% anaerobes (Porphyromonas 30.0%, Pyramidobacter 21.2%, Prevotella 8.5%, Filifactor 6.5%, Anaeroglobus 5.5%, and Solobacterium 3.1%) (Figure 2B).
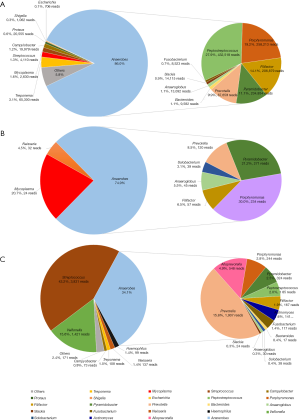
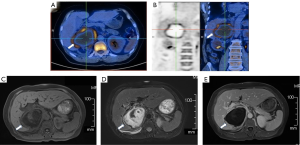
The patient was clinically relieved of back pain, and the inflammatory biomarkers, including the WBC count, CRP and ESR, were decreased after completing 16 days of combination antimicrobial therapy and drainage. Pus mNGS revealed dramatically decreased reads and anaerobic numbers (Figure 2C). Repeated upper abdominal enhanced MRI showed an obvious reduction in the size of the adrenal mass (Figure 3). The patient was transitioned to oral maintenance therapy consisting of faropenan at 0.2 g every 8 hours for eleven weeks. The follow-up lab examination of inflammatory biomarkers was normal, and the adrenal mass was reduced radiographically.
Discussion
Adrenal gland abscess is extremely rare in adults. Symptoms of cute adrenal abscess are fever, chills, abdominal pain. Some chronic infection only presents with symptoms of chronic adrenocortical hypofunction due to the disturbance of the hypothalamic-pituitary-adrenal axis. When patients present with complaints, such as abdominal or back pain; fever with elevated lab inflammatory markers; and underlying immunosuppression, such as HIV co-infection, immunosuppressant drug usage or recent surgical procedures, abscessation of the adrenal glands should be considered. The adrenal glands are retroperitoneal with a rich blood supply from several intraperitoneal vessels. The right adrenal gland is more susceptible to hemorrhage due to anatomical differences, accounting for approximately 70% of cases. This case also involved the right adrenal gland, but the patient was not obviously immunocompromised, as mentioned before.
The literature shows that pathogens in adrenal abscesses are diverse (2-4,6-10). Staphylococcus aureus and Escherichia coli are the most common bacteria in acute infection, while Tuberculosis, Nocardia, fungi and parasites are more common in chronic infection. We were amazed to find a polymicrobial infection with a large number of anaerobic bacteria in our case. The pathogenesis of this case was ambiguous. The microorganisms detected in our case were mostly from the human oral cavity, some of which have been reported to cause gingivitis, periodontopathy, and among other diseases (11-14) . Our patient had previously smoked for more than ten years and had poor oral hygiene, so we hypothesize that the pathogenic microorganisms might have originated from ectopic oral flora and disseminated to the right adrenal gland through the blood, although the patient underwent no dental procedures.
Anaerobic bacteria are common causes of infections, often involving polymicrobial flora, some of which can be serious and life-threatening. Because of their fastidious nature, anaerobes are rarely cultured and isolated, as they require specific inoculation methods and culture equipment. The results of anaerobic cultures are usually received after treatment decisions are made.
mNGS technology takes approximately 2 days to identify microorganisms, whereas culture-based methods take almost 2–7 days. Moreover, mNGS can detect unculturable or difficult-to-culture microorganisms. Unlike specific PCR, mNGS can identify multiple pathogens simultaneously with no prior assumptions (9). Rare cases have reported adrenal gland abscess caused by anaerobes, but no case of multiple anaerobes has been reported, suggesting that their limitations in traditional cultivation methods and that vigilance against anaerobe-induced infections is required.
This case suggests that mNGS technology has the significant advantages of obtaining rapid and accurate etiological diagnoses in suspected anaerobic infection, especially during co-infection with multiple microorganisms. mNGS can provide an important basis for choosing medication in clinical practice.
In conclusion, to our knowledge, this is the first report of an adrenal gland abscess primarily due to multiple anaerobes detected by mNGS analysis. mNGS technology provides rapid and accurate determination of pathogens and has promising value for detecting anaerobes and coinfection with multiple microorganisms in the future.
Acknowledgments
Funding: This work was supported by the BGI China (precision medical funding) and the 4th Three-year Action Plan for Public Health of Shanghai (Project No. 15GWZK0101).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arena F, Romeo C, Manganaro A, et al. Bilateral neonatal adrenal abscess. Report of two cases and review of the literature. Pediatr Med Chir 2003;25:185-9. [PubMed]
- Rumińska M, Witkowska-Sędek E, Warchoł S, et al. Adrenal abscess in a 3-week-old neonate - a case report. J Ultrason 2015;15:429-37. [Crossref] [PubMed]
- Urrutia A, Santesmases J, Benítez RM, et al. Adrenal gland abscess due to Streptococcus pneumoniae. J Infect 2010;60:88-9. [Crossref] [PubMed]
- Upadhyay J, Sudhindra P, Abraham G, et al. Tuberculosis of the adrenal gland: a case report and review of the literature of infections of the adrenal gland. Int J Endocrinol 2014;2014:876037. [Crossref] [PubMed]
- Spahn S, Helmchen B, Zingg U. Alveolar echinococcosis of the right adrenal gland: a case report and review of the literature. J Med Case Rep 2016;10:325. [Crossref] [PubMed]
- Jackson C, McCullar B, Joglekar K, et al. Disseminated Nocardia Farcinica Pneumonia with Left Adrenal Gland Abscess. Cureus 2017;9:e1160. [PubMed]
- Forbes JD, Knox NC, Ronholm J, et al. Metagenomics: the next culture-independent game changer. Front Microbiol 2017;8:1069. [Crossref] [PubMed]
- Hilton SK, Castro-Nallar E, Pérez-Losada M, et al. Metataxonomic and metagenomic approaches vs. Culture-based techniques for clinical pathology. Front Microbiol 2016;7:484. [Crossref] [PubMed]
- Jacob HJ. Next-generation sequencing for clinical diagnostics. N Engl J Med 2013;369:1557-8. [Crossref] [PubMed]
- Tachezy M, Simon P, Ilchmann C, et al. Abscess of adrenal gland caused by disseminated subacute Nocardia farcinica pneumonia. A case report and mini-review of the literature. BMC Infect Dis 2009;9:194. [Crossref] [PubMed]
- Stingu CS, Schaumann R, Jentsch H, et al. Association of periodontitis with increased colonization by Prevotella nigrescens. J Investig Clin Dent 2013;4:20-5. [Crossref] [PubMed]
- Bik EM, Long CD, Armitage GC, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J 2010;4:962-74. [Crossref] [PubMed]
- Aruni AW, Mishra A, Dou Y, et al. Filifactor alocis--a new emerging periodontal pathogen. Microbes Infect 2015;17:517-30. [Crossref] [PubMed]
- Roingeard C, Jaubert J, Guilleminault L. A large and unusual lung abscess with positive culture to Slackia exigua. Int J Infect Dis 2015;40:37-8. [Crossref] [PubMed]