
Pericardial fluid levels of growth differentiation factor 15 in patients with or without coronary artery disease: a prospective study
Introduction
Growth-differentiation factor-15 (GDF-15) was reported to be a novel transforming growth factor-β superfamily member (1). Elevated plasma levels of GDF-15 is associated with elevated clinical non-cardiovascular and cardiovascular morbidity; it plays a vital role in progression of heart diseases such as atrial fibrillation, coronary artery diseases and heart failure (2). The GDF15 protein is upregulated as part of the circulating anti-inflammatory cytokines within the cardiomyocyte in response to ischemia and injury (3). It is weakly expressed by cardiomyocytes under normal environmental and physiological conditions (4). In response to ischemia and oxidative stress, there is a significant rise in the expression level of GDF-15 (5). Therefore, it seems that this biomarker is of great importance in Coronary artery disease, which is involved with these above mechanisms (6).
Coronary artery disease (CAD) is the major cause of mortality in the society with elevating incidence in recent years. The pathological processes include chronic inflammation, endothelial dysfunction, oxidative stress and vascular calcification which together contribute to plaque formation, ending up with a cardiovascular event (7). It is a combination of different syndromes including acute coronary syndrome (ACS) and stable angina, arrhythmia, and heart failure (8).
Based on the data and information of several clinical trials, GDF-15 could be considered as a reliable biomarker in the examination of cardiac disease (9-11). It has a strong prognostic significance in predicting heart failure, coronary artery disease and atrial fibrillation (12).
Pericardium is well known for its protective content and contains a mixture of fluid which provides typically a microenvironment with proangiogenic cytokines secreted by cardiomyocytes (13). Studies with pericardial fluid are scarce mainly due to the fact that colleting pericardial fluid samples is of great difficulty (14). Pericardial fluid can be harvested by heart surgery. It can be a window for evaluating the pathophysiological processes of the heart (15). Mounting evidence suggests that vasoactive and cardioactive agents exist at high levels in the human pericardial fluid (16-18). However, to date, no analyzed data exist regarding the pericardial fluid GDF-15 in clinical patients with heart disease. Our study was aimed to make an evaluation on the pericardial fluid GDF-15 levels and their clinical diagnostic validity in the patients with or without ischemic heart disease, and further make an assessment on the relationship between pericardial GDF-15 and baseline biochemical variables and echocardiography parameters.
Methods
Patients population
A total of 42 patients participated in this observational, prospective clinical study. Patients undergoing open heart surgery in our Cardiovascular Surgery were consecutively registered. Patients were excluded as follows: primary and secondary cardiomyopathy, age <18 or >80 years, cardiac dilation and low LVEF (<45%), emergency operation, acute myocardial infarction within 30 days, malignant tumor, autoimmune disease or inflammatory disease.
Clinical data collection
Based on the clinical medical records, patients’ clinical data was collected comprehensively, such as sex, age, cardiovascular risk factors, and the LVEF parameters of transthoracic echocardiography. LVEF is a parameter of the percentage of blood ejecting the heart each time it contracts and was defined by the American Heart Association as a measurement of heart failure. From short-axis imaging, M-mode left ventricular ejection fraction based on the cubed method was equal to (end-diastolic volume end-systolic volume)/end-systolic volume.
Blood and pericardial fluid Biochemical analyses
The blood samples of enrolled patients were taken as the baseline reference on the second day after admission to the hospital. Many measurements were performed, including blood glucose, creatinine, CK-MB, cTnI and NT-proBNP. Blood collected from patients was stored in an anticoagulant tube. During the open heart procedure, following incision of the pericardium, undiluted pericardial fluid was immediately obtained prior to heparinization. All suspected blood-polluted pericardial fluid samples were excluded. Blood and pericardial fluid samples were centrifuged at 3,000 r/min for 15min and the supernatant were stored at −80 °C until analysis. Commercially available instruments and kits were used according to instructions
from the manufacturer. Biochemical measurements were performed using standard laboratory techniques. Plasma and pericardial fluid GDF15 concentrations were determined by quantitative sandwich enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, USA). The color intensity was measured with a multiwell spectrophotometer (BioTek, Winooski, VT, USA) at 450 nm.
Statistical analyses
Experimental data were analyzed by statistical software GraphPad Prism 7.0 (California, USA) and SPSS version 22.0 (Chicago, Illinois, USA). All of the continuous variables were shown as means ± Standard Error of Mean (SEM); Normality was tested using the Kolmogorov-Smirnov test. In all of the tests, a double-sided value of P<0.05 was considered to be statistically significant. Spearman’s correlation test was used to calculate the associations between pericardial GDF15 and other measured clinical laboratory parameters.
Results
Clinical characteristics of patients
Clinical and preoperative biochemical laboratory data of the 42 enrolled patients are listed in Table 1. The demographics and clinical presentations among patients in two groups are summarized. There was no statistically significant difference in clinical baseline presentations between the CAD and non-CAD group patients.
Full table
Pericardial fluid and plasma GDF15 levels in patients with or without CAD
Mean pericardial fluid GDF-15 levels were 677.8±77.2 and 617.4±76.2 pg/mL in CAD and non-CAD group respectively. Mean plasma GDF-15 levels were 1174.0±148.7 and 925.8±127.4 pg/mL in CAD and non-CAD Group respectively. As illustrated in Figure 1A, the concentration of GDF15 in plasma was significantly higher than the pericardial fluid GDF15 levels both in the CAD and non-CAD group (P<0.01). The pericardial fluid GDF15 levels was similar in both groups (P>0.05). Spearman’s correlation test was used to calculate the associations between pericardial GDF15 and other measured biochemical parameters. A positive correlation was found between pericardial fluid and plasma GDF15 concentrations in CAD group patients (R=0.53, P<0.01, Figure 1B). An obvious correlation was also observed between plasma and pericardial fluid GDF15 concentration in non-CAD patients (R=0.54, P<0.01, Figure 1C).

Associations between pericardial fluid GDF15 levels and baseline characteristics in CAD patients
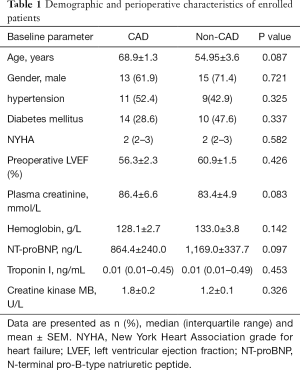
Associations between laboratory parameters and pericardial fluid GDF15 concentrations in CAD patients was tested by Spearman’s correlation test. A significant correlation was found between pericardial fluid GDF15 and circulating creatinine concentrations in CAD group patients (R=0.65, P<0.01, Figure 2A). Furthermore, a significant correlation was found between pericardial fluid and plasma NT-ProBNP levels in CAD patients (R=0.63, P<0.01, Figure 2B). However, no significant correlation was found between the pericardial fluid GDF15 and cTnI (R=−0.26, P >0.05).
Relationship between pericardial fluid GDF15 levels and baseline characteristics in non-CAD patients
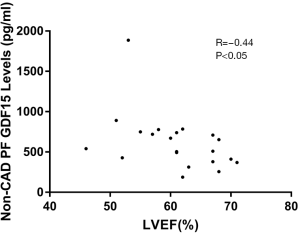
To document a relationship between pericardial fluid GDF15 concentrations and heart failure, we correlated LVEF with pericardial fluid GDF15 concentrations. In the CAD patients, correlation was not observed between pericardial fluid GDF15 levels and LVEF (R=−0.22, P>0.05). It is interesting that correlation was not observed between pericardial fluid GDF15 levels and plasma NT-ProBNP in non-CAD patients (R=0.36, R>0.05). Furthermore, an obvious significant association was observed between the pericardial fluid GDF15 levels and LVEF in non-CAD patients (R=−0.44; P<0.05, Figure 3). The pericardial fluid GDF15 concentrations reflected the cardiac function as evaluated by LVEF in non-CAD patients.
Discussion
The heart tissue produces many physiologically active substances such as cardiac derived hormones, cytokines and growth factors (19). It is not completely understood that how the mechanisms work when it is involved with substances synthesis, secretion, and metabolism (20). Currently, it has not been emphasized that which is the prominent pathway for the released substances from the heart (19,21). The traditional biochemical analysis of human PF showed that small molecules are similarly concentrated compared to plasma. However, some researchers revealing that PF is not only a simply ultrafiltrate of plasma and have potential diagnostic value for heart diseases (22).
This study demonstrated that the plasma GDF15 concentration was dramatically higher than the pericardial fluid in patients with or without coronary artery disease. Moreover, GDF levels in pericardial fluid were not significantly different between patients with ischemic and nonischemic heart disease. It revealed that we cannot distinguish patients with from those without CAD by pericardial fluid GDF15 only.
Biochemical markers are valuable and give prognostic information to cardiologists, including an established marker troponin and newer markers which are now finding their way into clinical decision-making (23). Some clinical research literatures also shown that plasma GDF15 has a significant association with myocardial injury (12,24,25). GDF15 has been well known to be elevated in patients with myocardial infarction and be a valuable biomarker of outcome in two clinical trials including patients with AMI (26,27). Our earlier study has reported that circulating GDF-15 can act as an effective biomarker for postoperative myocardial infarction in patients undergoing off pump CABG procedure (28).
To further demonstrate a correlation between pericardial fluid GDF15 levels and plasma biomarkers, in this study, we correlated baseline characteristics with pericardial fluid GDF15 levels. There was an obvious correlation between plasma and pericardial fluid GDF15 concentration both in the CAD and non-CAD group. This study is the first time to reveal pericardial fluid GDF15 in patients with heart disease. An obvious correlation was observed between pericardial fluid GDF15 levels and plasma NT-ProBNP in CAD patients. A positive correlation was also found between pericardial fluid GDF15 and plasma creatinine concentrations in CAD patients but not in non-CAD Patients. To our knowledge, these finding first reported GDF15 levels of pericardial fluid is associated with cardiac function and kidney function in patients with CAD.
It has been reported that the change of GDF15 levels could reveal both pathophysiological processes, as well cardiovascular inflammation (29). It is also demonstrated that higher plasma GDF15 concentrations in patients with chronic heart failure (30). GDF15 is induced in hypertrophic and dilated cardiomyopathy after volume overload, ischemia, and heart failure (30).
In the present study, even no obvious correlation was observed between pericardial fluid GDF15 levels and plasma NT-ProBNP in non-CAD patients, there was a positive correlation between pericardial fluid GDF15 and LVEF. We propose that it is valuable to identify and measure pericardial fluid GDF15 levels combined with traditional markers as a novel method to improve the accuracy of the traditional marker approach in future.
A few limitations deserve mention in this study. First of all, only the pericardial fluid GDF15 concentrations and clinical parameters were analyzed in this study, so to get the experimental data in a deeper level is very necessary when exploring the biological changes of pericardial fluid. Secondly, a relatively small sample size may decrease and fade the significance of correlation in this study. Thus, it is essential to have a wider range of participants join the study to verify that pericardial fluid GDF15 can be used as a clinical indicator for cardiac dysfunction.
Conclusions
This is the first time to demonstrate that the levels of GDF15 in plasma was significantly higher than the pericardial fluid both in the CAD and non-CAD group. In some degree, pericardial fluid GDF15 cannot distinguish patients with from those without CAD. Pericardial fluid GDF15 levels is associated with cardiac and kidney function in patients with coronary artery disease and might serve as a clinically validated role for predicting the complication and severity of CAD.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81671832, 81200093). Acknowledges support QNPY program of Ruijin Hospital (2019QNPY01011 Z Yuan).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the independent Medical Ethics Committee of the Ruijin Hospital, Shanghai Jiaotong University School of Medicine (2016095).
References
- Kempf T, Eden M, Strelau J, et al. The transforming growth factor-β superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006;98:351-60. [Crossref] [PubMed]
- Katus HA, Giannitsis E. Biomarker in cardiology. Clin Res Cardiol 2018;107:10-5. [Crossref] [PubMed]
- Brenière C, Meloux A, Pédard M, et al. Growth differentiation factor-15 (GDF-15) is associated with mortality in ischemic stroke patients treated with acute revascularization therapy. Front Neurol 2019;10:611. [Crossref] [PubMed]
- Kempf T, Wollert KC. Growth-differentiation factor-15 in heart failure. Heart Fail Clin 2009;5:537-47. [Crossref] [PubMed]
- Sharma A, Stevens SR, Lucas J, et al. Utility of Growth Differentiation Factor-15, A Marker of Oxidative Stress and Inflammation, in Chronic Heart Failure: Insights From the HF-ACTION Study. JACC Heart Fail 2017;5:724-34. [Crossref] [PubMed]
- Arkoumani M, Papadopoulou-Marketou N, Nicolaides NC, et al. The clinical impact of growth differentiation factor-15 in heart disease: a 2019 update. Crit Rev Clin Lab Sci 2020;57:114-25. [Crossref] [PubMed]
- Moreira DM, da Silva RL, Vieira JL, et al. Role of vascular inflammation in coronary artery disease: potential of anti-inflammatory drugs in the prevention of atherothrombosis. Am J Cardiovasc Drugs 2015;15:1-11. [Crossref] [PubMed]
- Ford TJ, Corcoran D, Berry C. Stable coronary syndromes: pathophysiology, diagnostic advances and therapeutic need. Heart 2018;104:284-92. [PubMed]
- Cotter G, Voors AA, Prescott MF, et al. Growth differentiation factor 15 (GDF‐15) in patients admitted for acute heart failure: results from the RELAX‐AHF study. Eur J Heart Fail 2015;17:1133-43. [Crossref] [PubMed]
- Hagström E, James SK, Bertilsson M, et al. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J 2016;37:1325-33. [Crossref] [PubMed]
- Wallentin L, Hijazi Z, Andersson U, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circulation 2014;130:1847-58. [Crossref] [PubMed]
- Wollert KC, Kempf T, Wallentin L. Growth differentiation factor 15 as a biomarker in cardiovascular disease. Clin Chem 2017;63:140-51. [Crossref] [PubMed]
- Beltrami C, Besnier M, Shantikumar S, et al. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther 2017;25:679-93. [Crossref] [PubMed]
- Ege T, Us MH, Cikirikcioglu M, et al. Analysis of C-reactive protein and biochemical parameters in pericardial fluid. Yonsei Med J 2006;47:372-6. [Crossref] [PubMed]
- Fujita M, Komeda M, Hasegawa K, et al. Pericardial fluid as a new material for clinical heart research. Int J Cardiol 2001;77:113-8. [Crossref] [PubMed]
- Tambara K, Fujita M, Miyamoto S, et al. Pericardial fluid level of heart-type cytoplasmic fatty acid-binding protein (H-FABP) is an indicator of severe myocardial ischemia. Int J Cardiol 2004;93:281-4. [Crossref] [PubMed]
- Foglio E, Puddighinu G, Fasanaro P, et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int J Cardiol 2015;197:333-47. [Crossref] [PubMed]
- Sahoo S, Mathiyalagan P, Hajjar RJ. Pericardial fluid exosomes: a new material to treat cardiovascular disease. Mol Ther 2017;25:568-9. [Crossref] [PubMed]
- Xiang F, Guo X, Chen W, et al. Proteomics analysis of human pericardial fluid. Proteomics 2013;13:2692-5. [Crossref] [PubMed]
- Fancello L, Monteil S, Popgeorgiev N, et al. Viral communities associated with human pericardial fluids in idiopathic pericarditis. PLoS One 2014;9:e93367. [Crossref] [PubMed]
- Hoit BD. Anatomy and Physiology of the Pericardium. Cardiol Clin 2017;35:481-90. [Crossref] [PubMed]
- Imazio M, Biondo A, Ricci D, et al. Contemporary biochemical analysis of normal pericardial fluid. Heart 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Stein EA, Raal F. Several protein biomarkers, including cardiac troponin T, cardiac troponin I, B-type natriuretic peptide, C-reactive protein and apolipoprotein AI, are widely employed in the evaluation of cardiovascular disease. Several of such potential biomarkers, or their multiscores, have been assessed over the last years for the prediction of cardiovascular risk but only a few of them have been validated for. Cardiovasc Drugs Ther 2016;30:101-8. [Crossref] [PubMed]
- Chiotoroiu A-L, Buicu C-F, Neagu C, et al. Recent Advances in Biomarker Discovery—from Serum to Imaging-based Biomarkers for a Complex Assessment of Heart Failure Patients. J Interdiscip Med 2016;1:125-30. [Crossref]
- Schernthaner C, Lichtenauer M, Wernly B, et al. Multi‐Biomarker Analysis in Patients with Acute Myocardial Infarction. Eur J Clin Invest 2017;47:638-48. [Crossref] [PubMed]
- Kempf T, Björklund E, Olofsson S, et al. Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J 2007;28:2858-65. [Crossref] [PubMed]
- Nursalim A, Suryaatmadja M, Panggabean M. Potential clinical application of novel cardiac biomarkers for acute myocardial infarction. Acta Med Indones 2013;45:240-50. [PubMed]
- Yuan Z, Li H, Qi Q, et al. Plasma levels of growth differentiation factor-15 are associated with myocardial injury in patients undergoing off-pump coronary artery bypass grafting. Sci Rep 2016;6:28221. [Crossref] [PubMed]
- Wollert KC, Kempf T. Growth differentiation factor 15 in heart failure: an update. Curr Heart Fail Rep 2012;9:337-45. [Crossref] [PubMed]
- Kempf T, von Haehling S, Peter T, et al. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol 2007;50:1054-60. [Crossref] [PubMed]


