
Optimized clinical randomized controlled trials designed for biomarker-guided antibiotics stewardship
We recently read a very interesting article of Hellyer’s research team, which was recently published in Lancet Respir Med (1). The authors performed VAPrapid2 trial to determine whether measurement of bronchoalveolar lavage fluid (BALF) IL-1β and IL-8 could effectively and safely improve antibiotic stewardship in patients with clinically suspected ventilator-associated pneumonia (VAP) and they concluded that antibiotic stewardship was not improved by IL-1β and IL-8 test. However, we consider some issues should be further analyzed and discussed.
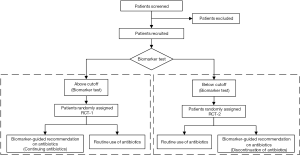
Firstly, we think there are some flaws in the design should be concerned. Patients were randomly assigned (1:1) BALF IL-1β/IL-8 test guided recommendation on antibiotics (intervention) or routine use of antibiotics group (control). In intervention group, clinicians were advised to consider continuing or discontinuing antibiotics according to the concentrations of BALF IL-1β and IL-8. Eventually, the IL-1β and IL-8 result was high in 64 patients and low in 17 patients. In our opinion, these 17 patients would have longer antibiotic-free days since they were consider discontinuing antibiotics, comparing to control group (routine use of antibiotics) while the recommendation to discontinue antibiotics was followed only in 4 (24%) patients, which was too low to achieve the certain conclusion. In this study, the authors observed no significant differences between intervention and control groups for all other secondary outcomes, including the serious adverse events. Actually, we suggest that eligible patients could be conducted the biomarker test, such as BALF IL-1β and IL-8 test, firstly and be divided into in “above cutoff” or “below cutoff” groups. And in each group, patients were randomly assigned intervention (“consider continuing antibiotics” in above cutoff group and “consider discontinuation of antibiotics” in below cutoff group, separately) or control group (routine use of antibiotics). The design flow is detailed in Figure 1. Even if not, the authors should perform the sub-group analysis and follow-up in the intervention group with biomarker-guided recommendation according to IL-1β and IL-8 compared with control group in this study at least.
Secondly, the combination of "almost perfect" indicators of BALF IL-1β and IL-8 has a perfect sensitivity (100%) and perfect negative predictive value (1.0, 95% CI: 0.92–1.0). The Youden index is only 0.44 (Youden index = sensitivity + specificity –1) (2,3). High positive rate would be always paralleled with high false positive rate and more patients would be misdiagnosed for VAP with continued using antibiotics. In this study, only 21% patients (17/81) are below the cutoff of IL-1β/IL-8. In this condition, it might underestimate the effectiveness of the bio-marker in guiding antibiotic stewardship. Hence, optimal combined diagnosis of biomarkers for VAP in critical care patients should be explored and validated.
Acknowledgments
Funding: This work was supported by grants from the National key Research & Development plan of Ministry of Science and Technology of the People’s Republic of China (Grant no. 2018YFC1314900 and Grant no. 2018YFC1314901), Youth Program of National Natural Science Foundation of China (Grant no. 81703301).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hellyer TP, McAuley DF, Walsh TS, et al. Biomarker-guided antibiotic stewardship in suspected ventilator-associated pneumonia (VAPrapid2): a randomised controlled trial and process evaluation. Lancet Respir Med 2020;8:182-91. [Crossref] [PubMed]
- Conway Morris A, Kefala K, Wilkinson TS, et al. Diagnostic importance of pulmonary interleukin-1beta and interleukin-8 in ventilator-associated pneumonia. Thorax 2010;65:201-7. [Crossref] [PubMed]
- Hellyer TP, Morris AC, McAuley DF, et al. Diagnostic accuracy of pulmonary host inflammatory mediators in the exclusion of ventilator-acquired pneumonia. Thorax 2015;70:41-7. [Crossref] [PubMed]


