Establishing M1 stage subdivisions by incorporating radiological features and Epstein-Barr virus DNA for metastatic nasopharyngeal carcinoma
Introduction
Survival outcomes of nasopharyngeal carcinoma (NPC) have considerably improved attributable to new diagnostic and therapeutic patterns (1,2). However, distant metastasis, accounting for 10% at initial diagnosis (synchronous metastatic NPC, smNPC) or 15–30% at progression (metachronous metastatic NPC, mmNPC) (3,4), still remains major failure despite radiosensitivity and chemosensitivity of NPC, and is accompanied with multidisciplinary therapies and unsatisfactory outcomes (5).
The American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system outlines the predominant guidelines for survival evaluation along with treatment decision in NPC in clinical practice. In the 8th edition of AJCC Cancer Staging Manual, mNPC (whether mmNPC or smNPC) is defined as an inclusive M1 classification (6). Nevertheless, overall survival (OS) of mNPC patients showed prognostic discrepancy, and controversial related factors were proposed. Distant metastases with liver and bone involvements contributed to unfavorable outcomes, whereas lung oligometastases showed superior survival (7,8). Besides, concepts of oligometastasis (an exclusive subset of patients with limited number and site of metastatic deposits along with better outcomes derived from metastatic entirety) were different (3,8,9). Prolonged disease control was reported in a patient with bone-involved oligometastatic NPC, indicating a probably steady state maintained in specific mNPC patients (10). Hence, an effective and precise M1 stage subdivision strategy was warranted.
In addition to anatomic factors, plasma titer of Epstein-Barr virus deoxyribonucleic acid (EBV DNA) is a powerful concomitant indicator correlated with tumor burden for World Health Organization (WHO) types II and III NPC. Detection of this tumor marker has demonstrated ultrasensitivity in NPC screening, risk stratification, and disease surveillance. Incorporation of EBV DNA in TNM staging system for T and N exhibited better results of predicting survival outcomes (11,12). EBV DNA has been used as an effective biomarker to discover metastasis during surveillance apart from image examination. Quantification of plasma EBV DNA was also interpreted as an independent prognosticator of great value in mNPC (13), but relevant studies to further elucidate their relationship were lacking. Besides, roles of critical anatomic features and EBV DNA regarding M1 subclassification in smNPC and mmNPC were not in consensus (3,8,14).
Therefore, we conducted a retrospective study in a large cohort of patients with mNPC and established recursive partitioning analysis (RPA)-based stratification of M1 stage (15). We attempted to combine both anatomic and biological factors for better prognostic evaluation, and thus explore better treatment strategy to aid clinical management and guide clinical trials.
Methods
Study population
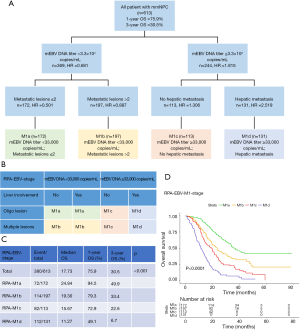
This retrospective study enrolled 1,007 patients with pathologically-proven mNPC from Sun Yat-sen University Cancer Center (SYSUCC), composed of 817 mmNPC patients consecutively diagnosed from November 2010 to August 2018 and 190 smNPC patients from January 2012 to August 2017. The mmNPC patients were initially confirmed with non-mNPC from April 2009 to December 2015 and subsequently underwent standard therapy. Patients with WHO type I NPC, a history of other malignancies, distant metastases that appeared during treatment or those who had incomplete clinical information were excluded (Figure 1).
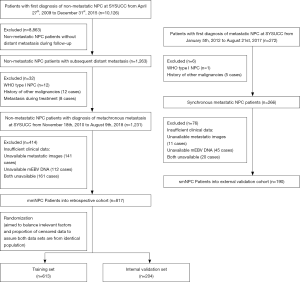
To validate the statistical process and manifest the applicability of the results, the total mmNPC cohort was randomized into a training set (n=613) and an internal validation set (n=204), in a proportion of 3:1 (Figure 1). Randomization balanced the irrelevant factors and censored data to avoid bias and guarantee, to some extent, that both data sets originated from an identical population. In addition, we defined an smNPC validation cohort (n=190) for validation to determine compatibility of the results (Figure 1).
All procedures were approved by the Institutional Review Board and the Ethics Committee of SYSUCC (approval number: YB2019-22), and the need for informed consent was waived by the ethics review boards. The authenticity of the article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the approval RDD number as RDDA2019001044.
Diagnosis of metastasis
All patients underwent routine evaluations including history taking, physical examination, hematology and biochemistry profiling, fiberoptic nasopharyngoscopy, and conventional radiography for assessing general conditions (1). If imaging studies (chest radiography and/or abdominal ultrasound and/or skeletal scintigraphy) indicates probability of metastasis, further information was obtained via additional evaluations to make definitive diagnoses, including ultrasound, computed tomography (CT), MRI, centesis, or pathological biopsy for the suspicious lesions, or fluorodeoxyglucose (18F) (FDG) positron emission tomography and CT (PET/CT) for the overall checkup (16).
As for metastasis of NPC, bone, lung, liver and distant lymph nodes were most frequently involved organs (14), and disseminations of other organs were classified as one category with rare occurrence. We also focused on the number of organs and lesions, and stratified patients into three groups respectively (1, 2 and >2 organs/lesions). All imaging data were determined according to Respond Evaluation Criteria in Solid Tumors (RECIST) guidelines (17). All patients were restaged by two experienced radiation oncologists specializing in head and neck cancers according to the AJCC staging system (8th edition) for their initial diagnosis (6), with disagreements resolved by consensus.
Definition of plasma EBV DNA at diagnosis of metastasis (mEBV DNA)
Prior to post-metastatic treatment, biological information (EBV DNA) was also collected. mEBV DNA was interpreted as EBV DNA titer measured within an interval of 2 weeks around the day when metastases were determined and before treatment. Detailed information was described in Supplementary file 1.
Treatment and follow-up
With respect to salvage therapy of mNPC, considering performance state and individual preference, clinicians carried out diverse approaches including chemotherapy, targeted therapy, or local treatment for eliminating confined metastatic lesion (radiotherapy, surgical operation, ablation or embolism). Platinum-based palliative chemotherapy was most extensively implemented in clinical practice. Oral chemotherapeutics like capecitabine functioned as general alternatives for patients who were intolerant to intravenous therapy. Other chemotherapeutic regimes were also applied. For smNPC, loco-regional radiotherapy for primary nasopharyngeal focus was an alternative choice.
Patients underwent routine examinations throughout and following the course of post-metastatic treatment. The primary end-point was OS, defined as the time interval measured from the date of identifying metastases to death from any causes, or to the latest date of follow-up (December 31st, 2018), for surviving patients or ones lost to follow-up.
Statistical analysis
We first analysed basic clinical characteristics of patients in the total mmNPC, training, internal validation and smNPC validation cohorts, using chi-squared test for categorical variables and Kruskal-Wallis test for continuous variables. OS was calculated using Kaplan-Meier method and compared by the log-rank tests. Univariable and multivariable Cox regression analyses were performed to identify significant characteristics for survival outcomes and to calculate hazard ratios (HRs). The cut-off value of mEBV DNA was determined by receiver operating characteristic (ROC) analyses. All tests with two-sided P value <0.05 were considered statistically significant.
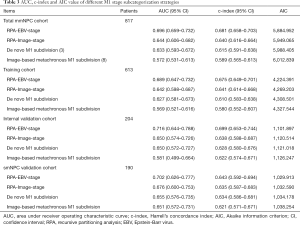
We explored different M1 stage subdivisions via RPA modeling methods from training cohort-initially based purely on significant metastatic radiological characteristics (termed RPA-Image-stage: M1A, M1B, and M1C), and subsequently incorporated mEBV DNA to establish M1 subdivisions (termed RPA-EBV-stage: M1a, M1b, M1c, and M1d). Subdivision strategies were validated in the internal validation set (mmNPC) and smNPC validation cohort. We then calculated area under ROC curve (AUC), Harrell’s concordance index (c-index), and Akaike information criterion (AIC) to evaluate the efficacy of different models, including two models of previous studies (3,8), to determine the optimal one. Cox regression analyses were used to explore treatment modalities according to the proposed RPA-EBV-stage.
All statistical analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA) or R version 3.5.1 (http://www.r-project.org/).
Results
Clinicopathological characteristics
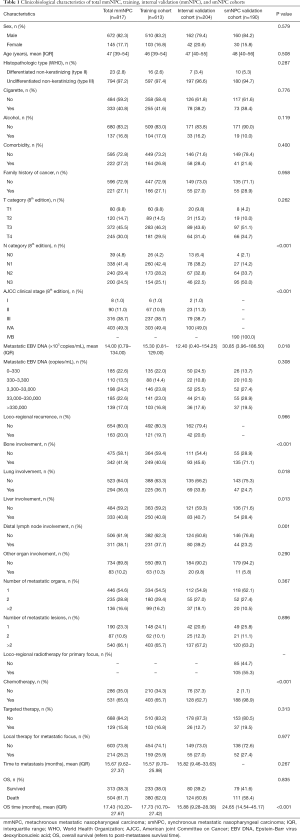
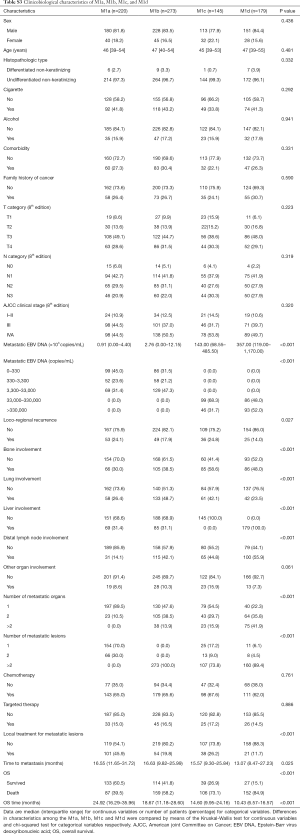
Detailed clinicopathological characteristics of patients are presented in Table 1 (baseline and metastatic characteristics) and Table S1 (therapeutic characteristics at first diagnosis of non-mNPC for mmNPC cohorts). Demographic characteristics were balanced among different cohorts. Higher proportion of N3 category, higher concentration of mEBV DNA, and different pattern of organ involvement were found in smNPC cohort.
Full table
Full table
Univariable and multivariable analyses of recognizing significant factors
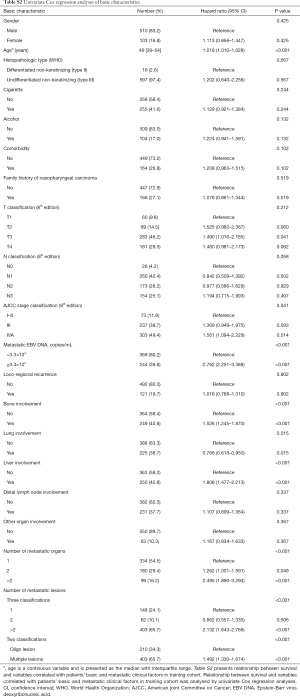
With univariable Cox regression analyses (Table S2), we observed significant differences between single organ involved and groups of multiple metastatic organs (>2) [P<0.001, HR 2.495 (95% CI: 1.890–3.294)]; group of two organs also seemed separate [P=0.049, HR 1.262 (95% CI: 1.001–1.591)]. With respect to number of metastatic lesions, multiple lesions (>2) seemed distinctive from the single lesion group [P<0.001, HR 2.132 (95% CI: 1.643–2.766)] while the group with two lesions did not show any difference [P=0.506, HR 0.862 (95% CI: 0.557–1.335)]. Thus, we defined “oligo lesion” as involvement of “≤2 metastatic lesions”. Corresponding Kaplan-Meier survival curves are presented in Figure S1.
Full table
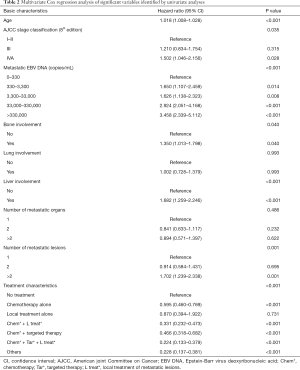
Through univariable (Table S2) and multivariable Cox regression analyses (Table 2), age (P<0.001) and initial AJCC clinical stage (8th edition) (P=0.035) were found important basic characteristics for OS. Bone involvement [bone involvement vs. no bone involvement: P=0.040, HR 1.350 (95% CI: 1.013–1.798)], liver involvement [liver involvement vs. no liver involvement: P<0.001, HR 1.682 (95% CI: 1.259–2.246)] and the number of metastatic lesions [n=2 vs. n=1: P=0.695, HR 0.914 (0.584–1.431); n>2 vs. n=1: P=0.001, HR 1.702 (95% CI: 1.239–2.338)] were identified as significant predictors with regard to metastatic radiological features. Lung involvement and number of metastatic organs (P=0.993 and 0.486 respectively) were no longer significant in the multivariable analyses.
Full table
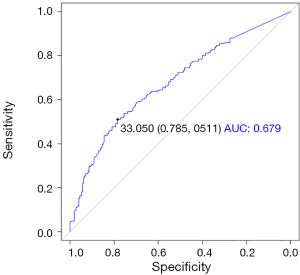
We performed ROC analyses and determined the cut-off value of mEBV DNA as 33,000 copies/mL (Figure S2); therefore, the population was divided into high mEBV (mEBV DNA ≥33,000 copies/mL) and low mEBV (mEBV DNA <33,000 copies/mL) groups. mEBV DNA was confirmed as important prognostic indicators (P<0.001) in multivariable Cox regression analyses (Table 2).
RPA- generated risk stratifications with or without incorporating mEBV DNA
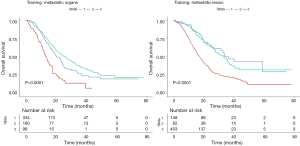
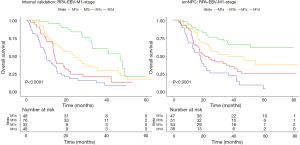
Next, we used RPA algorithm to subdivide M1 stage based on metastatic radiological characteristics, which ultimately subcategorized into three risk strata with divergent outcomes for OS (RPA-Image-stage, Figure 2; log-rank test P<0.001). Bone involvement did not enter the model. Corresponding 1-year (M1A to M1C: 90.1%, 77.2%, 57.9%) and 3-year OS rates (M1A to M1C: 46.3%, 31.0%, 11.2%) were significantly different (Figure 2C). Elevated mortality risk implicated by higher risk strata was confirmed when adjusted for age, initial clinical stage and treatment modality in RPA-Image-stage [M1B vs. M1A, HR 1.646 (95% CI: 1.269–2.133), P<0.001; M1C vs. M1A, HR 3.411 (95% CI: 2.620–4.439), P<0.001].
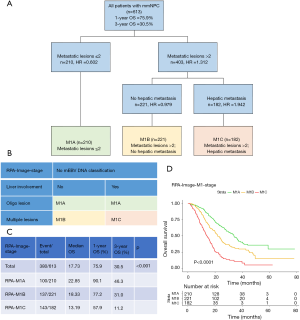
Then, mEBV DNA was integrated into the former M1 risk strata to establish a sound and improved M1 subcategorization (RPA-EBV-stage, Figure 3; log-rank test P<0.001). This biological marker was noted as a significant prognosticator for patients with identical image-based stage. The ultimate RPA-EBV-stage (Figure 3A,B) comprised four conclusive substages: M1a (low mEBV + oligo lesion), M1b (low mEBV + multiple lesions), M1c (high mEBV + no liver involvement), and M1d (high mEBV + liver involvement). Corresponding 1-year OS rates for M1a, M1b, M1c and M1d were 94.5%, 79.3%, 72.9%, and 49.1%; and corresponding 3-year OS rates were 49.9%, 33.4%, 22.6%, and 6.7%, respectively (Figure 3C, log-rank test P<0.001). Adjusted multivariable analysis illustrated an elevated mortality rate in higher risk group [M1b vs. M1a, HR 1.839 (95% CI: 1.366–2.475), P<0.001; M1c vs. M1a, HR 2.999 (95% CI: 2.178–4.131), P<0.001; RPA-M1d vs. RPA-M1a, HR 5.572 (95% CI: 4.097–7.578), P<0.001].
Validation and evaluation of different M1 subcategorization
Compared with M1 subcategorizations based merely on radiological characteristics, RPA-generated M1 subdivisions incorporating mEBV DNA presented a better performance with higher AUC and higher c-index together with lower AIC (Table 3). Furthermore, when compared with models proposed by previous studies (3,8), it also demonstrated a superior prognostic value for OS (Table 3). In addition, this M1 stage subcategorization also exhibited favorable compatibility in internal validation and smNPC validation cohort with best prognostications (Table 3 and Figure S3). The AUC, c-index and AIC value of RPA-EBV-stage in training cohort were 0.689, 0.675 and 4,224.391. Therefore, we believed that RPA-EBV-stage was a robust M1 stage subdivision strategy.
Full table
Novel risk stratification correlated with treatment outcomes by different therapeutic strategies
We then carried out subgroup analysis to explore the relationship between different therapeutic strategies and the proposed RPA-EBV-stage for mmNPC patients. Clinicobiological features were balanced in these four subgroups (Table S3). Detailed treatment modalities of four subgroups were presented in Table S4.
Full table

Full table
Next, we examined the interaction between our risk stratification and efficacy of the various treatment modalities (Table S5, chemotherapy alone as reference). For patients in M1a, we observed a superiority of chemotherapy combined with local treatment of metastatic lesion [without or with targeted therapy: HRChem*+L treat* =0.499 (95% CI: 0.259–0.960), P=0.037; HRChem*+Tar*+L treat* =0.389 (95% CI: 0.154–0.982), P=0.046] over chemotherapy alone. Patients who underwent aggressive treatment (combination of chemotherapy, targeted therapy and local treatment) in M1b had a lower mortality risk [HRChem*+Tar*+L treat* =0.384 (95% CI: 0.154–0.958), P=0.040], and group of chemotherapy combined with local treatment also seemed to be advantageous [HRChem*+L treat* =0.597 (95% CI: 0.352–1.012), P=0.055, marginally significant]. Treatment benefits from chemotherapy plus targeted therapy (without or with local treatment) over chemotherapy alone was observed both in M1c [HRChem*+Tar* =0.440 (95% CI: 0.203–0.956), P=0.038; HRChem*+Tar*+L treat* =0.367 (95% CI: 0.159–0.847), P=0.019] and M1d [HRChem*+Tar* =0.535 (95% CI: 0.294–0.975), P=0.041; HRChem*+Tar*+L treat* =0.147 (95% CI: 0.036–0.604), P=0.008].

Full table
Discussion
To our best knowledge, this is the first large-scale study to combine mEBV DNA to M1 stage subdivision in mmNPC and smNPC patients, and to evaluate individualized management. It substantiated several prominent findings. First, bone involvement, liver involvement and number of metastatic lesions were notable anatomic predictors for post-metastatic OS in mNPC patients. Apart from conventional radiological features, mEBV DNA appeared to be a remarkable independent prognosticator in mNPC. Next, we established an RPA-generated M1 stage risk stratification system incorporating mEBV DNA, which outperformed the simply image-based models with higher AUC, c-index and lower AIC in mmNPC and smNPC cohort. Third, we analyzed different therapeutic modalities in subgroups, and found that compared with chemotherapy alone, addition of local treatment may benefit low-risk patients, whereas high-risk patients might yield benefit from inclusion of targeted therapy.
Nowadays AJCC TNM staging system divides M category into M0 and M1 stages without detailed subdivisions. However, previous studies revealed considerable survival discrepancy in mNPC patients (8,10). With development and individualization of therapeutic modality, the catch-all denotation of M1 stage did not meet the current need of clinical works and researches for mNPC. On account of addressing this clinical problem, we conducted this large-scale retrospective study on 1,007 metastatic patients. Considering regular follow-up and comprehensive image studies of metachronous metastasis, we chose mmNPC patients as training set and validated the model in both mmNPC and smNPC patients. Clinical as well as biological indices were comprehensively analyzed, and bone involvement, liver involvement as well as multiple metastatic lesions were found to be adverse radiological prognosticators of OS, which was identical to what Shen et al. put forward (8).
EBV DNA, derived from tumor cells and thought to be biological surrogate of occult metastasis, was discussed for its substantial diagnostic and prognostic value for mNPC (18-20). Previous studies aimed to verify its function in survival prediction of mNPC, and disparate statistical methods such as Cox regression analyses or nomograms were applied (21,22). However, most studies did not clarify its value due to limited population, and some previous models were impractical for clinical use. Moreover, considering fluctuation of EBV DNA titers resulted from post-metastatic treatments, absence of specific measuring time of mEBV DNA might bring about bias and obscure its function. Thus, we first defined mEBV DNA, stipulating EBV DNA measuring time. This biological indicator manifested a significant prognostic ability (P<0.001).
Then we introduced a novel risk stratification strategy by RPA modeling method, and confirmed superior performance in the models integrating mEBV DNA to the simply image-based ones either in mmNPC or smNPC patients. Besides, we investigated the interaction between this biomarker and anatomic metastatic characteristics. For low mEBV subgroup (M1a + M1b) with limited occult metastasis, extent of macroscopic metastasis (manifested by number of metastatic lesions) plays a considerable part in predicting OS. Liver involvement acted as a significant indicator in high mEBV group (M1c + M1d), demonstrating burden of hematogenous metastasis. All these characteristics can be obtained simply by image examination and liquid biopsy tracking in clinical practice, which could help subsequent decision making.
Interestingly, results originated from the training cohort (mmNPC) showed great compatibility in an smNPC validation cohort, and the definition of “oligo lesion” raised in this article was same as what Zou et al. found in smNPC patients (3). Nevertheless, some previous studies revealed difference between mmNPC and smNPC patients, like OS or anatomic traits (14,21). Whether these two groups of patients are distinct still needs further exploration.
In patients with mNPC, multiple therapeutic patterns are applied substantially based on individual judgments of physicians. A randomized phase 3 trial of two platinum-based chemotherapeutic combinations in patients with recurrent or mNPC (GEM20110714 trial) reported gemcitabine plus cisplatin regime with superior survival (23). This regime was established as first-line palliative chemotherapy for mNPC patients. In addition, patients with confined metastasis undergo local treatment like operation, radiotherapy or ablation in most cases, aiming to achieve complete remission. However, we still lack a reliable strategy to stratify metastatic patients and guide clinical individualized administration. Adjusted for age, initial TNM clinical stage and therapeutic modality, RPA-EBV-stage presented a favorable capability of survival prediction (3-year OS of M1a to M1d: 49.9%, 33.4%, 22.6%, and 6.7%, respectively; P<0.001). Beyond prognostication, this M1 stage subcategorization strategy possesses the potential to guide treatment of mmNPC patients. Through subgroup analyses, we found that combination of different therapeutic patterns may confer a better prognosis (Table S5). Previous studies showed beneficial outcomes of oligometastatic patients undertaking local therapy (24,25). We observed superior survival outcomes in patients with local treatment in combination with palliative chemotherapy, to the ones who received chemotherapy alone, especially for oligometastatic patients (M1a: P<0.05; M1b: this is marginally significant). Therefore, we consider that patients with limited occult and macroscopic metastasis yield greatest benefit from addition of local treatment. Despite relevant scientific achievement in basic researches, clinical trials failed to prove drug efficacy of targeted therapy in mNPC (26-28). While in this study, groups with chemotherapy combined with targeted therapy presented advantageous prognosis for high mEBV subgroup. We infer that patients with high load of mEBV DNA are recommended to undertake intensive systemic therapy (inclusion of targeted therapy), or to participate in clinical trials. Since high-risk group was recognized, comparative intensive treatment strategies were advised to specific groups of patients and this risk stratification was thought to support better administration in clinical practice as well as provide evidence for population identification in clinical trials.
Our study has several potential limitations. First, all included patients were from a single cancer center. Nevertheless, this proposed RPA-EBV-stage was validated in the internal validation set and in an smNPC validation cohort with good performance. Second, EBV was treated as a dichotomous variable in our final model, which probably loses some of its prognostic ability. Third, results of the quantitative plasma EBV DNA were not harmonized. Therefore, validation of this prognostic scale and harmonization of EBV DNA analysis should be key issues for future prospective studies.
Conclusions
In conclusion, mEBV DNA was of great prognostic value for mNPC patients. The RPA-generated M1 stage subdivisions incorporating mEBV DNA presented better accuracy in survival prognostication of mNPC and can be considered to have appreciable clinical application.
Supplementary
The methodology of detecting metastatic Epstein-Barr virus (EBV) DNA
At 2 weeks around diagnosis of metastasis and before post-metastatic treatment, samples of peripheral blood (3 mL) were collected from each patient in an ethylenediaminetetraacetic acid (EDTA) tube, and centrifuged at 1,600 ×g for 15 min to isolate plasma and peripheral blood cells (PBC). DNA was extracted using the QIAamp Blood Kit (Qiagen, Hilden, Germany) and stored at −80 °C until further processing. A total of 500 µL plasma samples were used for DNA extraction per column, and a final elution volume of 50 µL was used to elute the DNA from the extraction column.
The concentration of EBV DNA in the plasma was measured using a real-time quantitative polymerase chain reaction (PCR) assay targeting the BamH I-W region of the EBV genome. The sequences of the forward and reverse primers were 5'-GCCAG AGGTA AGTGG ACTTT-3' and 5'-TACCA CCTCC TCTTC TTGCT-3', respectively. A dual fluorescently-labelled oligomer, 5'-(FAM) CACAC CCAGG CACAC ACTAC ACAT (TAMRA)-3' served as the probe. All relevant sequence data for the EBV genome were obtained from the GenBank sequence database.
Acknowledgments
We sincerely thank Yiducloud Technology Ltd., Beijing, China for assisting in part of data searching and processing on the big-data intelligence platform.
Funding: This work was supported by the National Natural Science Foundation of China (grant number 81872463); the Special Support Program of Sun Yat-sen University Cancer Center (grant number 16zxtzlc06); the Planned Science and Technology Project of Guangdong Province (grant number 2019B020230002); the Natural Science Foundation of Guangdong Province (grant number 2017A030312003); the Health & Medical Collaborative Innovation Project of Guangzhou City, China (grant number 201803040003); the Innovation Team Development Plan of the Ministry of Education (grant number IRT_17R110); and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project) (grant number B14035).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were approved by the Institutional Review Board and the Ethics Committee of SYSUCC (approval number: YB2019-22), and the need for informed consent was waived by the ethics review boards.
References
- Lee AW, Ma BB, Ng WT, et al. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J Clin Oncol 2015;33:3356-64. [Crossref] [PubMed]
- Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer 2017;36:90. [Crossref] [PubMed]
- Zou X, You R, Liu H, et al. Establishment and validation of M1 stage subdivisions for de novo metastatic nasopharyngeal carcinoma to better predict prognosis and guide treatment. Eur J Cancer 2017;77:117-26. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Chan AT, Gregoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii83-5. [Crossref] [PubMed]
- Amin MB, Edge SB, greene FL, et al. AJCC Cancer Staging Manual, 8th ed. New York, NY: Springer, 2018.
- Hui EP, Leung SF, Au JS, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Cancer 2004;101:300-6. [Crossref] [PubMed]
- Shen L, Li W, Wang S, et al. Image-based Multilevel Subdivision of M1 Category in TNM Staging System for Metastatic Nasopharyngeal Carcinoma. Radiology 2016;280:805-14. [Crossref] [PubMed]
- Tian YH, Zou WH, Xiao WW, et al. Oligometastases in AJCC stage IVc nasopharyngeal carcinoma: A subset with better overall survival. Head Neck 2016;38:1152-7. [Crossref] [PubMed]
- Khot A, Love C, Garg MK, et al. Long-Term Disease Control in a Patient With Recurrent Bone-Only Oligometastatic Nasopharyngeal Carcinoma. J Clin Oncol 2016;34:e25-6. [Crossref] [PubMed]
- Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer 2019;125:79-89. [Crossref] [PubMed]
- Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006;24:5414-8. [Crossref] [PubMed]
- Lin JC, Chen KY, Wang WY, et al. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol 2001;19:2607-15. [Crossref] [PubMed]
- Shen LJ, Wang SY, Xie GF, et al. Subdivision of M category for nasopharyngeal carcinoma with synchronous metastasis: time to expand the M categorization system. Chin J Cancer 2015;34:450-8. [Crossref] [PubMed]
- Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 2015;33:836-45. [Crossref] [PubMed]
- Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013;31:2861-9. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Wang WY, Twu CW, Chen HH, et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res 2010;16:1016-24. [Crossref] [PubMed]
- Hsu CL, Chang KP, Lin CY, et al. Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck 2012;34:1064-70. [Crossref] [PubMed]
- You R, Liu YP, Lin M, et al. Relationship of circulating tumor cells and Epstein-Barr virus DNA to progression-free survival and overall survival in metastatic nasopharyngeal carcinoma patients. Int J Cancer 2019;145:2873-83. [Crossref] [PubMed]
- Zeng Z, Shen L, Wang Y, et al. A nomogram for predicting survival of nasopharyngeal carcinoma patients with metachronous metastasis. Medicine (Baltimore) 2016;95:e4026. [Crossref] [PubMed]
- Jiang R, You R, Pei XQ, et al. Development of a ten-signature classifier using a support vector machine integrated approach to subdivide the M1 stage into M1a and M1b stages of nasopharyngeal carcinoma with synchronous metastases to better predict patients' survival. Oncotarget 2016;7:3645-57. [PubMed]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. [Crossref] [PubMed]
- Rusthoven CG, Lanning RM, Jones BL, et al. Metastatic nasopharyngeal carcinoma: Patterns of care and survival for patients receiving chemotherapy with and without local radiotherapy. Radiother Oncol 2017;124:139-46. [Crossref] [PubMed]
- Li W, Bai Y, Wu M, et al. Combined CT-guided radiofrequency ablation with systemic chemotherapy improves the survival for nasopharyngeal carcinoma with oligometastasis in liver: Propensity score matching analysis. Oncotarget 2016;8:52132-41. [PubMed]
- Lim WT, Ng QS, Ivy P, et al. A Phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res 2011;17:5481-9. [Crossref] [PubMed]
- Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol 2005;23:3568-76. [Crossref] [PubMed]
- Chua DT, Wei WI, Wong MP, et al. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck 2008;30:863-7. [Crossref] [PubMed]