
Protective effects of curcumin against neuroinflammation induced by Aβ25-35 in primary rat microglia: modulation of high-mobility group box 1, toll-like receptor 4 and receptor for advanced glycation end products expression
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by intracellular neurofibrillary tangles and extracellular deposition of amyloid-beta (Aβ) (1). Microglia, the resident immune cells in the brain, are the first line of defense to protect the CNS from injury and invading pathogens (2). Activated microglia is induced by Aβ release of several proinflammatory mediators and can induce neurotoxicity and promote the pathological cascade of AD (3,4).
The high mobility group box-1 protein 1 (HMGB1), a non-histone chromosomal binding protein released from damaged cells or cells under stressful conditions, can directly induce tissue damage and promote the release of proinflammatory mediators (5,6). HMGB1-mediated inflammatory responses through receptor for advanced glycation end products (RAGE) and toll-like receptor 4 (TLR4), which have been implicated in memory impairments in AD (7-9). Preventing the production of HMGB1 or HMGB1-mediated inflammatory responses may function as a therapeutic target for AD.
Curcumin, a yellow-colored ingredient of the spice turmeric, was extracted from the rhizome of curcuma longa (turmeric). Curcumin has powerful antioxidant, anti-inflammatory and anti-amyloidogenic properties that may have neuroprotective effects in AD (10-13). Also, recent studies have suggested that curcumin protects against propionibacterium acne (14) or concanavalin A-induced liver injury (15,16) through inhibition of HMGB1 cytoplasmic translocation and expression. Besides, curcumin could decrease the levels of HMGB1, TLR4 or TLR2 expression in fibrogenesis hepatocytes (17) and lipopolysaccharide-treated human endothelial cells (18). Whether curcumin could be an effective agent for inhibiting inflammation by suppressing the production of HMGB1 or HMGB1-mediated inflammatory responses in Aβ-activated microglia is still unclear.
In this study, we examined whether curcumin inhibits Aβ25-35-induced neuroinflammation in primary rat microglia by suppressing HMGB1, TLR4, and RAGE expression.
Methods
All animal experiments were conducted following the animal protocols approved by the Animal Ethics Committee of the Second Affiliated Hospital & Yuying Children Hospital of Wenzhou Medical University.
Chemicals
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), Aβ25-35, and curcumin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rabbit polyclonal anti-TLR4, anti-RAGE antibodies, and rabbit monoclonal anti-GAPDH antibody were from Cell Signaling Technology (Beverly, USA). Rabbit monoclonal anti-HMGB1 antibody was from Epitomics, Inc. (Burlingame, CA, USA). Horseradish peroxidase (HRP)-conjugated goat-anti-rabbit secondary antibody was purchased from Kangcheng Biotech Co. Ltd. (Shanghai, China). Tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) ELISA kits were from R&D Systems (Minneapolis, MN, USA). High mobility group protein B1 (HMGB1) ELISA kit was from Shino-Test Co., (Kyoto, Japan).
Preparation of Aβ25-35
Aβ25-35 was dissolved in DMSO at 10 mM and stored at −20 °C until use. The stock solution was diluted with deionized distilled water to a proper concentration and incubated at 37 °C for 4 days for aggregation before use. The final concentration of DMSO was less than 0.01%, which did not affect cell viability.
Aβ25-35 treatment and curcumin administration
Microglia were treated with Aβ25-35 50 µM for 24 h to prove a toxic effect. Curcumin 10 µM was administrated 1 h before Aβ25-35 treatment.
Cell culture
Sprague Dawley rats were purchased from the Experimental Animal Center of Wenzhou University. As previously described (19), primary microglia were prepared from the cerebral cortices of 1- to 3-day-old Sprague Dawley rats. Briefly, cortical tissue was triturated into single cells and incubated in DMEM holding 10% FBS. After centrifugation, cells were suspended and plated in poly-D-lysine coated 75 cm2 flasks (1×107 cells/flask) for 9–10 days at 37 °C in a humidified 95% air and 5% CO2 incubator. Microglia were then detached from the flasks by mild shaking for 2 h at 200 rpm. Reserved supernatant was harvested and filtered through a nylon mesh to remove astrocytes. Cells were plated in six-well plates (2×106 cells/well) for 1 h and were washed to remove unattached cells before use.
Pheochromocytoma (PC12) cells were obtained from the Institute of Biochemistry and Cell Biology, SIBS, CAS (Shanghai, China). PC12 Cells were planted in 25 cm2 flasks and incubated in DMEM holding 10% FBS at 37 °C in a humidified 95% air and 5% CO2 incubator.
Cell viability assay
Cell viability was assessed by MTT assay as described previously (20). In brief, PC12 Cells were seeded in 96 well plates at a density of 5×103 cells/well in serum-free DMEM and allowed to adhere at 37 °C for 24 h. After treatment with a conditioned medium for 24 h, 20 µL MTT reagent (final concentration, 2.5 mg/mL) was added to each well and the plate was incubated at 37 °C for 4 h. The medium was aspirated off, and 150 µL DMSO was added to each well. The optical density of each well was read at 570 nm using a microplate reader (ELX808, Biotek, Winooski, VT, USA).
Western blot analysis
Proteins were loaded and separated in 10% SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk for 2 h at room temperature and followed by incubation with primary antibodies overnight at 4 °C. The membranes were then incubated with the HRP-conjugated secondary antibodies for 2 h at room temperature, and the expression of the protein was visualized by the enhanced chemiluminescence reagent (ECL) using an Imaging System (ChemiDoc MP, Bio-Rad).
Enzyme-linked immunosorbent assay (ELISA)
The levels of TNF-αIL-1β (R&D Systems, Minneapolis, MN, USA), and HMGB1 (Shino-Test Co., Kyoto, Japan) in the culture supernatants were measured with ELISA kits according to the manufacturer’s instructions.
Statistical analysis
Results were presented as mean ± standard deviation. Statistical analyses were performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Differences among experimental groups were evaluated by one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
Curcumin inhibits HMGB1 production in Aβ25-35-stimulated microglia
We supposed that curcumin could be an effective agent for inhibiting neuroinflammation by suppressing the production of HMGB1. Our preliminary results showed that the concentration of HMGB1 in the culture medium increased significantly after the Aβ25-35 stimulation. Pretreatment with curcumin significantly decreased the concentration of HMGB1 in the culture medium (Figure 1A). Curcumin also significantly inhibited HMGB1 expression in microglia treated with Aβ25-35 (Figure 1B). These results suggest that curcumin inhibits HMGB1 production in Aβ25-35-stimulated microglia.
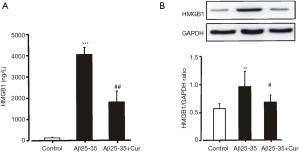
Curcumin inhibits HMGB1-mediated inflammatory responses in Aβ25-35-stimulated microglia
HMGB1-mediated inflammatory responses through RAGE and TLR4 had been implicated in memory impairments and cell death in AD (7-9). To explore whether curcumin inhibits HMGB1-mediated inflammatory responses in Aβ25-35-stimulated microglia, we first examined the effects of curcumin on the expressions of RAGE and TLR4. Aβ25-35 induced a significantly increased expression of RAGE and TLR4 (Figure 2A,B). Pretreated with curcumin could significantly inhibit RAGE and TLR4 expression. The levels of IL-1β and TNF-α in the culture medium were significantly reduced by curcumin (Figure 2C,D). These results suggest that curcumin inhibits HMGB1-mediated inflammatory responses in Aβ25-35-stimulated microglia by decreasing the expression of RAGE and TLR4.
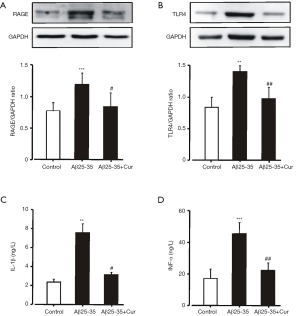
Curcumin inhibits the toxicity of Aβ25-35-activated microglia to neurons
Because our results suggest that curcumin inhibits HMGB1 production and HMGB1-mediated inflammatory responses in Aβ25-35-stimulated microglia. Next, we investigated whether curcumin inhibits the toxicity of activated microglia to neurons. We used an MTT assay to examine the effect of curcumin on the toxicity of Aβ25-35-activated microglia PC12 cells. The cell viability was significantly decreased after treatment with conditioned medium from Aβ25-35-treated microglia. However, when PC12 cells were incubated in conditioned medium from microglia treated with curcumin and Aβ25-35, the cell viability was significantly increased (Figure 3). Our data suggest that curcumin inhibits the toxicity of Aβ25-35-activated microglia to neurons.
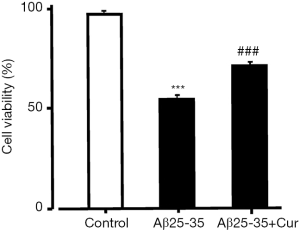
Discussion
Because of its potent antioxidant, anti-inflammatory and anti-amyloidogenic properties, curcumin, a natural ingredient extracted from Curcuma longa, has shown potential therapeutic effect for AD (10-13). HMGB1 can directly induce neuronal damage and promote the release of inflammatory mediators, which is a risk factor for AD (5-9), as a non-histone chromosomal binding protein released from damaged cells or cells under stressful conditions.
Takata et al. found that the level of HMGB1 was significantly increased, and HMGB1 expression was colocalized in senile plaques associated with microglia in the tested AD rat brain samples (21). They further performed another two studies and found that extracellular HMGB1 released from dying neurons could decrease microglial Aβ clearance, which enhanced the neurotoxicity of Aβ (22,23). Thus, the inhibition of HMGB1 production may be a therapeutic strategy for AD. However, so far, the effect of curcumin on HMGB1 expression in Aβ-activated microglia is still unclear.
Recent studies have indicated that curcumin could be an effective agent for inhibiting the HMGB1 cytoplasmic translocation and expression in the acute inflammatory liver injury model (14-16). Two earlier studies had shown the effects of curcumin pretreatment on concanavalin A-induced hepatitis in mice. Curcumin significantly suppressed the intrahepatic expression of HMGB1 by inhibiting the translocation from the nucleus to the cytoplasm (15,16). Gu et al. also found that curcumin treatment attenuated propionibacterium acnes-induced liver injury through reducing cytoplasmic expression of HMGB1 (14). Thus, we suspected that curcumin pretreatment could reduce the expression of HMGB1 in Aβ25-35-stimulated microglia. Indeed, our results showed that curcumin could significantly reduce the HMGB1 level in the culture medium and decrease the expression of HMGB1 in microglia stimulated with Aβ25-35.
Once released from the cytoplasm, extracellular HMGB1 can induce a pro-inflammatory response through binding to RAGE and TLR4 (7-9). Several recent studies had examined these effects of curcumin on HMGB1-mediated proinflammatory responses. In HMGB1-activated human umbilical endothelial cells, curcumin not only decreased the cell surface expression of TLR2 and TLR4 but also inhibited the levels of VCAM-1, ICAM-1, and E-selectin (18). Gu et al. found that propionibacterium acne treatment can increase the expression of TLR2, TLR4, and RAGE in mice liver. Curcumin treatment significantly reduced the expression of TLR4, but not of RAGE and TLR2 (14). However, the effects of curcumin on HMGB1-mediated proinflammatory responses have not been explored in Aβ25-35-stimulated microglia. In the present study, we found that Aβ25-35 induced significant increased expression of TLR4 and RAGE in microglia. Pretreated with curcumin could significantly inhibit the expression of TLR4 and RAGE. The proinflammatory cytokines such as TNF-α and IL-1β expression were also remarkably reduced by curcumin. These results indicated that curcumin inhibits HMGB1-mediated inflammatory responses in Aβ25-35-stimulated microglia likely in part by decreasing the expression of TLR4 and RAGE.
Because curcumin significantly inhibits HMGB1 production and HMGB1-mediated inflammatory responses in Aβ25-35- stimulated microglia. We last to examine whether curcumin inhibits the toxicity of activated microglia to neurons. Our result showed that curcumin protected neurons against toxicity induced by Aβ25-35-activated microglia, which had consistency with earlier studies (24,25). Yang et al. indicated that curcumin could protect hippocampal HT22 cells from toxicity mediated by erythrocyte lysis-treated microglia (24). Guo et al. also found that curcumin suppressed the production of proinflammatory mediators in HIV-1-gp120-stimulated microglia and protected cortical neurons against HIV-1-induced toxicity (25).
In summary, the present study supplies evidence that curcumin could effectively inhibit neuroinflammation induced by Aβ25-35 in primary rat microglia likely in part through suppressing the expression of HMGB1, TLR4, and RAGE. Our results add a new insight about the molecular mechanisms by which curcumin exerts anti-inflammatory effects against Aβ-activated microglia.
Acknowledgments
Funding: The current study was supported by grants from the National Natural Science Foundation of China (grant no. 81271204), Natural Science Foundation of Zhejiang Province (grant no.LY17H090015), program from Science and Technology Bureau of Wenzhou (grant no. 2017Y0596).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were conducted following the animal protocols approved by the Animal Ethics Committee of the Second Affiliated Hospital & Yuying Children Hospital of Wenzhou Medical University.
References
- Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis 2001;3:75-80. [Crossref] [PubMed]
- Wang MM, Miao D, Cao XP, et al. Innate immune activation in Alzheimer’s disease. Ann Transl Med 2018;6:177. [Crossref] [PubMed]
- Cai Z, Hussain MD, Yan LJ. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int J Neurosci 2014;124:307-21. [Crossref] [PubMed]
- Spangenberg EE, Green KN. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav Immun 2017;61:1-11. [Crossref] [PubMed]
- Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191-5. [Crossref] [PubMed]
- Gauley J, Pisetsky DS. The translocation of HMGB1 during cell activation and cell death. Autoimmunity 2009;42:299-301. [Crossref] [PubMed]
- Arancio O, Zhang HP, Chen X, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. Embo J 2004;23:4096-105. [Crossref] [PubMed]
- Buchanan MM, Hutchinson M, Watkins LR, et al. Toll-like receptor 4 in CNS pathologies. J Neurochem 2010;114:13-27. [PubMed]
- Mazarati A, Maroso M, Iori V, et al. High-mobility group box-1 impairs memory in mice through both toll-like receptor 4 and Receptor for Advanced Glycation End Products. Exp Neurol 2011;232:143-8. [Crossref] [PubMed]
- Goozee KG, Shah TM, Sohrabi HR, et al. Examining the potential clinical value of curcumin in the prevention and diagnosis of Alzheimer’s disease. Br J Nutr 2016;115:449-65. [Crossref] [PubMed]
- Massa F, Meli R, Morbelli S, et al. Serum neurofilament light chain rate of change in Alzheimer’s disease: potentials applications and notes of caution. Ann Transl Med 2019;7:S133. [Crossref] [PubMed]
- Lim GP, Chu T, Yang F, et al. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001;21:8370-7. [Crossref] [PubMed]
- Ye J, Zhang Y. Curcumin protects against intracellular amyloid toxicity in rat primary neurons. Int J Clin Exp Med 2012;5:44-9. [PubMed]
- Gu Q, Guan H, Shi Q, et al. Curcumin attenuated acute Propionibacterium acnes-induced liver injury through inhibition of HMGB1 expression in mice. Int Immunopharmacol 2015;24:159-65. [Crossref] [PubMed]
- Wang C, Nie H, Li K, et al. Curcumin inhibits HMGB1 releasing and attenuates concanavalin A-induced hepatitis in mice. Eur J Pharmacol 2012;697:152-7. [Crossref] [PubMed]
- Tu CT, Yao QY, Xu BL, et al. Curcumin Protects Against Concanavalin A-Induced Hepatitis in Mice Through Inhibiting the Cytoplasmic Translocation and Expression of High Mobility Group Box 1. Inflammation 2013;36:206-15. [Crossref] [PubMed]
- Tu CT, Yao QY, Xu BL, et al. Protective effects of curcumin against hepatic fibrosis induced by carbon tetrachloride: Modulation of high-mobility group box 1, Toll-like receptor 4 and 2 expression. Food Chem Toxicol 2012;50:3343-51. [Crossref] [PubMed]
- Kim DC, Lee W, Bae JS. Vascular anti-inflammatory effects of curcumin on HMGB1-mediated responses in vitro. Inflamm Res 2011;60:1161-8. [Crossref] [PubMed]
- Kim OS, Park EJ, Joe EH, et al. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem 2002;277:40594-601. [Crossref] [PubMed]
- Qian X, Cao H, Ma Q, et al. Allopregnanolone attenuates Aβ 25-35-induced neurotoxicity in PC12 cells by reducing oxidative stress. Int J Clin Exp Med 2015;8:13610-5. [PubMed]
- Takata K, Kitamura Y, Kakimura J, et al. Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem Biophys Res Commun 2003;301:699-703. [Crossref] [PubMed]
- Takata K, Kitamura Y, Tsuchiya D, et al. High Mobility Group Box Protein-1 Inhibits Microglial Aβ Clearance and Enhances Aβ Neurotoxicity. J Neurosci Res 2004;78:880-91. [Crossref] [PubMed]
- Takata K, Takada T, Ito A, et al. Microglial Amyloid-β1-40 Phagocytosis Dysfunction Is Caused by High-Mobility Group Box Protein-1: Implications for the Pathological Progression of Alzheimer's Disease. Int J Alzheimers Dis 2012;2012:685739.
- Yang Z, Zhao T, Zou Y, et al. Curcumin inhibits microglia inflammation and confers neuroprotection in intracerebral hemorrhage. Immunol Lett 2014;160:89-95. [Crossref] [PubMed]
- Guo L, Xing Y, Pan R, et al. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS One 2013;8:e70565. [Crossref] [PubMed]


