
Targeting the mitogen-activated protein kinase-mediated vascular smooth muscle cell remodeling by angiotensin II
Angiotensin II (Ang II) is a main effector peptide of the renin-angiotensin system that plays an important role in various cardiovascular diseases associated with vascular smooth muscle cell (VSMC) proliferation and migration, including hypertension, atherosclerosis and myocardial infarction (1). Ang II causes a rapid rise in blood pressure by stimulating Ang II type 1 receptor in arterial VSMC to contract. Ang II is also associated with the slow onset of hypertension by cardiac and vascular remodeling induced by cardiovascular cellular hypertrophy, proliferation and migration (2).
In the present issue of Ann Transl Med, Wang et al. report that cortistatin (CST) inhibited Ang II-induced VSMC proliferation and migration by suppressing extracellular signal-regulated kinase (ERK) 1/2, p38 mitogen-activated protein kinase (MAPK), c-Jun N-terminal kinase (JNK) and ERK5 signaling pathways (3) (Figure 1). Earlier studies have revealed that the Ang II-induced cellular responses are attributable, in part, to the phosphorylation of intracellular signaling molecules including the mitogen-activated protein (MAP) kinase family, i.e., ERK1/2, JNK (4). It has been reported that Ang II phosphorylates ERK1/2, JNK in VSMC (5,6). We also reported that Ang II stimulated ERK1/2, JNK and p38 MAPK activation in VSMC and Ang II caused cellular hypertrophy (7), migration (8) and proliferation (9). From these findings, it is currently obvious that Ang II stimulates VSMC proliferation and migration through the activation of MAP kinase pathways.
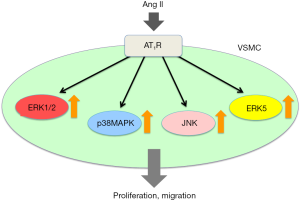
ERK5, also known as big MAP kinase 1, is a newly identified MAP kinase family member with a large COOH-terminal and a unique loop-12 sequence that shares the TEY activation motif with ERK1/2 but is activated by MAPK kinase 5 (MEK5) (10). ERK5 has been reported to be sensitive to osmotic (11) and oxidative stresses (12). In the present issue of Ann Transl Med, Wang et al. used Western blotting analysis with a phospho-specific antibody to show that Ang II activated ERK5 in VSMC. We have previously found that aldosterone (13) and platelet-derived growth factor (PDGF) (14), both stimulated ERK5 activation in VSMC. From these findings, it is clear that ERK5, as well as other classical MAP kinases, is activated by various neuro-humoral substances and causes cellular phenotypic modulations such as survival or proliferation. Previously, we found that ERK5 works as a survival factor of PC12 cells after oxidative insults (12). ERK5 is activated by osmotic stress in cultured rat mesangial cells induced by high glucose conditions in the medium which resulted in cellular proliferation (11).
Targeting the MAP kinases, including ERK5, may provide insights for the treatment of cardiovascular diseases associated with VSMC remodeling such as proliferation and migration.
Wang et al. showed that CST inhibited Ang II-induced MAP kinase activation and VSMC proliferation and migration (3). CST is a biological peptide which has protective effects for the cardiovascular system (15). Exploring the inhibiting compounds for MAP kinase activities is another strategy to prevent cardiovascular diseases associated with VSMC proliferation and migration. Previously, we found that JNK and p38 MAPK are sensitive to reactive oxygen species (ROS) because antioxidants, diphenyleneiodonium chloride (DPI) and ascorbic acid, both inhibited JNK and p38 MAPK activation by Ang II in VSMC (16). We also reported that quercetin, a bioflavonoid belonging to polyphenols, inhibited Ang II-induced VSMC hypertrophy through the inhibition of Shc/phosphatidylinositol 3-kinase/JNK signaling pathway (7). Therefore, antioxidative properties against ROS may be candidates for inhibition of MAP kinase activities in VSMC. In contrast to JNK and p38 MAPK, ERK1/2 seems to be insensitive to ROS because various antioxidants showed no effects on Ang II-induced ERK1/2 activation in VSMC (16). Since Wang et al. showed that CST inhibited Ang II-induced ERK1/2 activation, the inhibitory mechanism of CST may not be attributable to the antioxidative properties. As mentioned in the Discussion of their manuscript, GSK3β/β-catenin pathway may be involved (3). Due to this, further studies are needed to elucidate the precise signaling pathways of the ERK1/2, p38 MAPK, JNK, ERK5 that may be involved in the proliferation and migration of VSMCs. In addition, searching for the inhibitors of the MAP kinase family members may be promising area of research for the discovery of agents associated with cardiovascular diseases relevant to VSMC remodeling.
VSMC hypertrophy and proliferation are features of vascular remodeling through thickening of the medial layer of arterial wall. VSMC hypertrophy and proliferation causes the narrowing of the small arteries that consist of resistance vessels for blood flow. VSMC migration also induces a thickening of the arterial wall as well as having a role in angiogenesis. As well as direct counting of the cell numbers (9), [3H]-thymidine incorporation into the cells is often used to evaluate cell proliferation since thymidine is utilized in DNA synthesis (17). In Addition, colorimetric assay with CST-8 (9) or CCK8 (3) are also used for the measurement of many types of cell proliferation. Because leucine is a source for protein synthesis, [3H]-leucine uptake into the cells is commonly used as an indicator measurement of cellular hypertrophy in addition to the measurement of the protein contents of the cells (7). For measurement of VSMC migration, direct counting of migrated cells with a wound healing assay (18) or with a transwell cell migration assay (3) is frequently employed. Measurement of VSMC hypertrophy, proliferation and migration may be good indexes for the screening of drugs for cardiovascular diseases.
As an unsolved problem in the development of cardiovascular drugs, searching for agents against aortic dissection is a very important line of investigation (19-21). Although drugs for hypertension are the most effective for the prevention of aortic dissection by reducing high blood pressure, residual risk for rupture of an aneurysm still remains even after lowering the blood pressure. Another unsolved question is developing agents for diabetes mellitus related vascular diseases. We previously found that insulin-induced glucose uptake into the VSMC was inhibited by Ang II evoked ERK1/2 activation (22). Therefore, MAP kinase may be involved in the pathogenesis of diabetes mellitus-mediated vascular diseases including microangiopathy. Thus, exploring the future drugs for the prevention of aortic dissection and treatment of diabetes mellitus related vascular diseases are obvious next steps for further research.
Taken together, exploring the inhibitors of MAP kinases which are activated by Ang II stimulation in VSMC is a convincing strategy for the development of drugs for treatment of cardiovascular diseases in which VSMC remodeling may be involved such as proliferation and migration.
Acknowledgments
We thank Professor Francesco A. Bolstad, Dept. of Clinical English at Nara Medical University School of Medicine for editing and proof reading of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berk BC, Haendeler J, Sottile J. Angiotensin II, atherosclerosis, and aortic aneurysms. J Clin Invest 2000;105:1525-6. [Crossref] [PubMed]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 2000;52:639-72. [PubMed]
- Wang Y, Zhang X, Gao L, et al. Cortistatin exerts antiproliferation and antimigration effects in vascular smooth muscle cells stimulated by Ang II through suppressing ERK1/2, p38 MAPK, JNK and ERK5 signaling pathways. Ann Transl Med 2019;7:561. [Crossref] [PubMed]
- Schmitz U, Berk BC. Angiotensin II signal transduction: Stimulation of multiple mitogen-activated protein kinase pathways. Trends Endocrinol Metab 1997;8:261-6. [Crossref] [PubMed]
- Tsuda T, Kawahara Y, Ishida Y, et al. Angiotensin II stimulates two myelin basic protein/microtubule-associated protein 2 kinases in cultured vascular smooth muscle cells. Circ Res 1992;71:620-30. [Crossref] [PubMed]
- Viedt C, Soto U, Krieger-Brauer HI, et al. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol 2000;20:940-8. [Crossref] [PubMed]
- Yoshizumi M, Tsuchiya K, Kirima K, et al. Quercetin inhibits Shc- and phosphatidylinositol 3-kinase-mediated c-Jun N-terminal kinase activation by angiotensin II in cultured rat aortic smooth muscle cells. Mol Pharmacol 2001;60:656-65. [PubMed]
- Kyaw M, Yoshizumi M, Tsuchiya K, et al. Src and Cas are essentially but differentially involved in angiotensin II-stimulated migration of vascular smooth muscle cells via extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase activation. Mol Pharmacol 2004;65:832-41. [Crossref] [PubMed]
- Nagayama K, Kyotani Y, Zhao J, et al. Exendin-4 prevents vascular smooth muscle cell proliferation and migration by angiotensin II via the inhibition of ERK1/2 and JNK signaing pathways. PLoS One 2015;10:e0137960. [Crossref] [PubMed]
- Lee JD, Ulevitch RJ, Han J. Primary structure of BMK1: a new mammalian map kinase. Biochem Biophys Res Commun 1995;213:715-24. [Crossref] [PubMed]
- Suzaki Y, Yoshizumi M, Kagami S, et al. BMK1 is activated in glomeruli of diabetic rats and in mesangial cells by high glucose conditions. Kidney Int 2004;65:1749-60. [Crossref] [PubMed]
- Suzaki Y, Yoshizumi M, Kagami S, et al. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells. J Biol Chem 2002;277:9614-21. [Crossref] [PubMed]
- Ishizawa K, Izawa Y, Ito H, et al. Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension 2005;46:1046-52. [Crossref] [PubMed]
- Izawa Y, Yoshizumi M, Ishizawa K, et al. Big mitogen-activated protein kinase 1 (BMK1)/extracellular signal-regulated kinase 5 (ERK5) is involved in platelet-derived growth factor (PDGF)-induced vascular smooth muscle cell migration. Hypertens Res 2007;30:1107-17. [Crossref] [PubMed]
- Liu Y, Yin XH, Qi YF. Progress in biological effects of cortistatin. Sheng Li Ke Xue Jin Zhan 2009;40:219-24. [PubMed]
- Kyaw M, Yoshizumi M, Tsuchiya K, et al. Antioxidants inhibit JNK and p38 MAPK activation but not ERK1/2 activation by angiotensin II in rat aortic smooth muscle cells. Hypertens Res 2001;24:251-61. [Crossref] [PubMed]
- Yoshizumi M, Kim S, Kagami S, et al. Effect of endothelin-1 (1-31) on extracellular signal-regulated kinase and proliferation of human coronary smooth muscle cells. Br J Pharmacol 1998;125:1019-27. [Crossref] [PubMed]
- Kyotani Y, Zhao J, Tomita S, et al. Olmesartan inhibits angiotensin II-Induced migration of vascular smooth muscle cells through Src and mitogen-activated protein kinase pathways. J Pharmacol Sci 2010;113:161-68. [Crossref] [PubMed]
- Zhao J, Nishimura Y, Kimura A, et al. Chemokines protect vascular smooth muscle cells from cell death induced by cyclic mechanical stretch. Sci Rep 2017;7:16128. [Crossref] [PubMed]
- Zhao J, Ozawa K, Kyotani Y, et al. Azelnidipine inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch. PLoS One 2014;9:e102813. [Crossref] [PubMed]
- Ito S, Ozawa K, Zhao J, et al. Olmesartan inhibits cultured rat aortic smooth muscle cell death induced by cyclic mechanical stretch through the inhibition of the c-Jun N-terminal kinase and p38 signaling pathways. J Pharmacol Sci 2015;127:69-74. [Crossref] [PubMed]
- Izawa Y, Yoshizumi M, Fujita Y, et al. ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells. Exp Cell Res 2005;308:291-99. [Crossref] [PubMed]


