
The predictive value of baseline hepatic venous pressure gradient for variceal rebleeding in cirrhotic patients receiving secondary prevention
Introduction
Gastroesophageal variceal bleeding is one of the most serious complications of portal hypertension with high mortality in patients with cirrhosis (1). Hepatic venous pressure gradient (HVPG) has been widely validated as a strong prognostic factor for decompensation events in patients with cirrhosis (2,3); and has been recommended by guidelines to predict the presence of rebleeding. A baseline-HVPG higher than 20 mmHg was associated with a significantly higher early rebleeding rate within 1 week after acute variceal bleeding in patients with cirrhosis (3). However, for patients receiving secondary prevention, there still lacks a strong evidence showing whether baseline-HVPG could predict rebleeding. In these patients, the results and utility of the HVPG measurement face additional confounding factors. Compared to patients with compensated cirrhosis or undergoing first acute variceal bleeding, patients receiving secondary prevention for variceal bleeding have usually experienced a relatively long decompensated period. Besides, patients with cirrhosis receiving secondary prevention are usually in a more intense condition of hyperdynamic circulation (4), which brings instability in hemodynamics and rapid exacerbation of disease, resulting in higher risk of death (3). Also, at the time of HVPG measurement, these patients have usually initiated secondary prevention, which may influence the hemodynamics and measurement of HVPG to a varied extent (5). An HVPG influenced by interventions may be insufficient to reflect the actual disease condition. In this study, we aim to investigate the predictive performance of baseline-HVPG for variceal rebleeding in cirrhotic patients who received secondary prevention.
Methods
Study population
We retrospectively enrolled consecutive patients with cirrhosis in Shandong Provincial Hospital between October 2010 and July 2018. The patient inclusion criteria were: (I) received secondary prevention of variceal rebleeding [endoscopic variceal ligation (EVL) combined with non-selective beta-blocker (NSBB) or EVL alone when there was an NSBB contraindication]; (II) had HVPG measurement before the second episode of variceal bleeding; (III) had at least 1-year follow-up after HVPG measurement unless the occurrence of rebleeding. Patients who had HVPG measurement within 5 days prior to EVL for secondary prevention of variceal rebleeding were also included. To avoid influence of EVL on the accuracy of HVPG, patients who had HVPG measurement within 48 hours after EVL were excluded (6). Patients with Child-Pugh class C were also excluded. This study was approved by the Medical Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University (No. 2019-51) and the patient informed consent was achieved.
NSBB treatment and EVL procedure
For NSBB treatment, either carvedilol or propranolol was used. Carvedilol was started at an initial dose of 6.25 mg once-daily and adjusted gradually to the maximum tolerated dose (12.5 mg once-daily), keeping heart rate >55 beats per minute and systolic blood pressure >90 mmHg. Propranolol was started at an initial dose of 10 mg three times-daily an adjusted gradually to the maximum tolerated dose (40 mg three times-daily), keeping heart rate >55 beats per minute and systolic blood pressure >90 mmHg. EVL was performed using commercial multiband devices (Wilson-COOK Medical Inc., North Carolina, USA) under sedation with propofol. Varices were ligated from the cardia to the oral side. The ligation was conducted at 4-week intervals until variceal eradication. The varices were considered eradicated when they had disappeared or could not be grasped and banded by the ligator.
HVPG measurement
HVPG measurements were performed using balloon catheters with a pressure transducer at the tip (Edwards Lifesciences, Irvine, California, USA). A zero measurement was performed before the transjugular catheterization. The right hepatic vein was chosen for measurements when feasible. Under circumstances of stenosis or vein-to-vein shunt in the right hepatic vein, the middle hepatic vein was chosen. The free hepatic venous pressure was measured at the chosen hepatic vein close to the inferior vena cava. Then, the chosen hepatic vein was occluded completely by the inflated balloon and the wedged hepatic venous pressure was measured. Recording of results was continued until the pressure reached a plateau. All measurements were performed in triplicate and the average value was taken. HVPG was determined by subtracting the free hepatic venous pressure from the wedged hepatic venous pressure.
Statistical analysis
Continuous variables were shown as mean and standard deviation (SD) or median and interquartile range (IQR) while categorical variables were shown as frequencies (%). Chi-square test and Fisher’s exact test was applied for comparison of rebleeding rate between groups. Time depending receiver operating characteristic curve (ROC) was employed to evaluate the time-dependent predictive performance of baseline-HVPG for rebleeding. Univariate and multivariate Cox models were used to calculate hazard ratio (HR) and 95% confidence interval (CI) of rebleeding for patients with different HVPG at different time points. For the multivariate Cox model, HVPG, platelet count, Model for End-stage Liver Disease score and acceptance of NSBB were included. HRs were calculated at day 60, 120, 180, 270 and 365 after HVPG measurement taking HVPG <12 mmHg as reference standard. HVPG was stratified into four groups: HVPG <12 mmHg, 12≤ HVPG <16 mmHg, 16≤ HVPG <20 mmHg and HVPG ≥20 mmHg, labeled as 1, 2, 3, and 4, respectively. We performed tests for liner trend by entering the ordered HVPG categories as a continuous variable in the Cox models. Linear trend test was applied for significance at different time points. Kaplan-Meier analysis with log-rank test was used for inter-group assessment. All levels of significance were set at two-sided 5% level. All analyses were performed using SPSS 20.0 IBM (IBM Corp., Armonk, NY, USA) and R 3.5.3 (R Project for Statistical Computing, Vienna, Austria).
Results
Patients
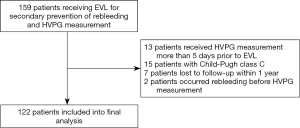
A total of 122 patients with cirrhosis who received secondary prevention of variceal rebleeding and baseline-HVPG measurement were included. Flow chart for the study enrollment is summarized in Figure S1. Besides EVL, 85 patients received either propranolol or carvedilol. Patients were followed up for 1 year unless the presence of rebleeding episode. Within 1 year, rebleeding occurred in 22 out of 122 patients (18.0%). Clinical characteristics of the whole studied cohort and rebleeding cases are summarized in Table 1 and Table S1.
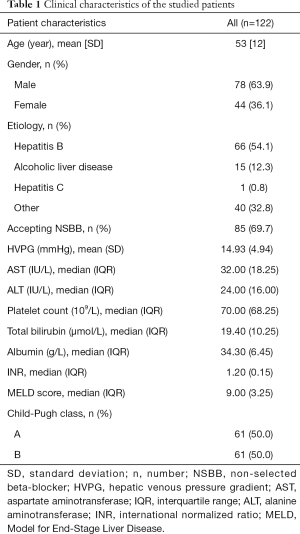
Full table
Full table
Occurrence of short-term and long-term rebleeding in patients with different HVPG levels
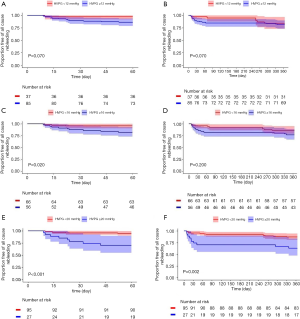
We analysed the rebleeding rate in patients with different HVPG levels and time points. Within 60 days after HVPG measurement, there was no significant differences in rebleeding rate between patients with HVPG <12 mmHg (n=37) and patients with HVPG ≥12 mmHg (n=85) (5.4% vs. 15.2%, P=0.106). Also, the rebleeding rate within 1 year had no difference between the two groups (16.2% vs. 18.8%, P=0.927). Kaplan-Meier analysis achieved similar results (Figure 1A,B). The cut-off values of 16 and 20 mmHg were also studied. Within 60 days after HVPG measurement, patients with HVPG ≥16 mmHg (n=56) showed significant higher rebleeding rate compared to patients with HVPG <16 mmHg (n=66) (17.9% vs. 4.5%, P=0.021). However, within 1 year after HVPG measurement, number of patients rebled in the two groups was 9 (13.6%) vs. 13 (23.2%) (P=0.256), respectively, with no significant differences observed. Again, validated by Kaplan-Meier analysis (Figure 1C,D). For patients with HVPG <20 mmHg (n=95), the rebleeding rate within 60 days and 1 year were both significantly lower than that of patients with HVPG ≥20 mmHg (n=27) (5.2% vs. 29.6%, P=0.001 and 12.6% vs. 37%, P=0.008, respectively). Besides, survival curve showed significant differences between two groups at both two aforementioned time points (Figure 1E,F). However, it should be noted that the rebleeding rate since 60 days showed no significant difference between two groups (7.8% vs. 10.5%, P=0.655) because in patients with HVPG ≥20 mmHg, most of the rebleeding (80%) occurred within 60 days. In summary, the results suggested that in patients who had received secondary prevention, HVPG ≥12 mmHg might not appropriate for predicting short-term (within 60 days) rebleeding while HVPG ≥16 or ≥20 mmHg indicated higher rate of short-term rebleeding. However, the above three cut-off values all seemed insufficient for predicting long-term (60 days after HVPG measurement) rebleeding.
Time-dependent predictive value of baseline-HVPG for rebleeding
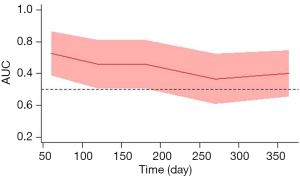
To clarify the time-dependent predictive value of baseline-HVPG for rebleeding, time-depending ROC analysis was performed. Area under the ROC curve (AUC) showed a decreasing trend by time. As shown by time-dependent ROC curve, the AUCs of predicting rebleeding using baseline-HVPG at day 60, 120, 180, 270 and 365 were 0.727 (95% CI: 0.585–0.868), 0.660 (95% CI: 0.506–0.815), 0.660 (95% CI: 0.506–0.815), 0.566 (95% CI: 0.407–0.726), and 0.601 (95% CI: 0.453–0.748), respectively (Figure 2).
Rebleeding risk at different time points in patients with stratified baseline-HVPG
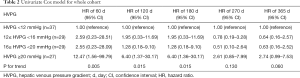
Univariate and multivariate Cox analysis were performed to show the relative rebleeding risk of patients stratified by baseline-HVPG at different time points. For univariate Cox model (Table 2), HRs for rebleeding (vs. HVPG <12 mmHg) within 60 days since HVPG measurement were 2.59 (95% CI: 0.23–28.51) for 12≤ HVPG <16 mmHg, 2.55 (95% CI: 0.23–28.09) for 16≤ HVPG <20 mmHg, and 12.47 (95% CI: 1.56–99.79) for HVPG ≥20 mmHg (P for trend =0.005). However, within 365 days, the respective HRs for the aforementioned three groups were 0.64 (95% CI: 0.16–2.57), 0.63 (95% CI: 0.16–2.52) and 2.74 (95% CI: 0.99–7.53) (P for trend =0.060).
Full table
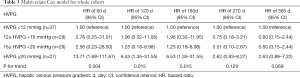
As shown by multivariate Cox model adjusted by platelet, albumin, Model for End-stage Liver Disease score and acceptance of NSBB, a similar decreased risk stratification efficacy of baseline-HVPG for rebleeding was observed (Table 3). HRs for rebleeding (vs. HVPG <12 mmHg) within 60 days since HVPG measurement were 2.78 (95% CI: 0.25–31.01) for 12≤ HVPG <16 mmHg, 2.56 (95% CI: 0.23–28.50) for 16≤ HVPG <20 mmHg and 13.71 (95% CI: 1.68–111.67) for HVPG ≥20 mmHg (P for trend =0.004). And HRs for rebleeding within 365 days since HVPG measurement were 0.60 (95% CI: 0.15–2.44), 0.60 (95% CI: 0.15–2.44) and 2.63 (95% CI: 0.89–7.22) for the aforementioned three groups. P for trend failed to reach statistical significance at day 270 (P for trend =0.129) and 365 (P for trend =0.069).
Full table
Discussion
As an indirect reflection of portal pressure, HVPG filters the confounding factors of the central venous system and thus serves as an accurate parameter for assessing portal hypertension (7). HVPG has already been well demonstrated to be a strong prognostic factor in cirrhotic patients with portal hypertension. An HVPG ≥10 mmHg is seen as clinically significant portal hypertension and combined with higher risks of developing varices and bleeding (2). Patients with an HVPG higher than 16 mmHg face increased mortality (8-10) and bleeding risk (11) while an HVPG above 20 mmHg is predictive of failure to control bleeding, early rebleeding and death ascribed to acute variceal hemorrhage (3,12).
In general, it is agreed that the higher the patients’ HVPG is, the higher the risk of bleeding they face. However, there exists little evidence on the predictive value of a single baseline-HVPG measurement for long-term rebleeding in patients with portal hypertension receiving secondary prevention. Current evidences provided by previous studies concentrate mainly on overall prognosis of patients within the compensated stage. In these studies, patients were followed-up for several years and thus robust evidences were provided for the predictive performance of baseline-HVPG (9,13). There are also several studies investigated the rebleeding predictive effect of HVPG in decompensated patients. An HVPG higher than 20 mmHg was proved to indicate higher risk of treatment failure or early rebleeding within 5 days (14,15), 1 week (3) or 6 weeks (16) in patients with cirrhosis who received different therapies for treatment of acute variceal bleeding. Only one study investigated the long-term prognosis of these patients with different HVPG (11). In this study by Li et al, although significant difference on rebleeding rate was reached taking HVPG threshold as 16 and 20 mmHg, the sample size is rather small and no data was provided on the timepoints of rebleeding for patients with different HVPG (11). Hence, there lacks a strong evidence on the rebleeding predictive effect of a single baseline-HVPG in patients with cirrhosis receiving secondary prevention of variceal rebleeding. Consistent to previous studies, we observed a higher overall rebleeding rate in patients with an HVPG ≥20 mmHg compared to those with an HVPG lower than 20 mmHg. Nevertheless, most rebleeding events occurred during the first 60 days of follow-up; and there was no difference (P=0.655) in rebleeding rate between the two groups from the 60th day to the 365th day after measurement of HVPG, suggesting a poor long-term predictive efficacy of baseline-HVPG. Our study demonstrated that in patients with cirrhotic portal hypertension receiving secondary prevention, a single baseline-HVPG has only limited long-term predictive effect.
Current studies support the role of HVPG for long-term prediction of bleeding in patients with compensated cirrhosis and short-term prediction of rebleeding in patients undergoing the first acute bleeding episode (3,9,13,14-16). However, compared to patients of compensated stage or underwent recent acute variceal hemorrhage, the portal pressure of patients receiving secondary prophylaxis faces more influential factors. Acute bleeding episodes and the following decompensation period may induce increment of HVPG and unstable hemodynamics, leading to shortened survival (14,17). Despite the fact that patients with compensated cirrhosis may also undergo spontaneous HVPG change which influences prognosis (18), it’s reasonable to believe that this process may be even more intense and rapid in decompensated patients, weakening the predictive efficacy of baseline-HVPG. Interventions performed before HVPG measurement could also be a source of inaccurate estimation of disease condition. Although the widely-applied EVL has been proved that it would not cause a sustained rise of HVPG, other interventions like sclerotherapy and transjugular intrahepatic portosystemic shunt long-termly influence HVPG to a different extend (6,19). Even for patients receiving EVL as monotherapy, results may still be compromised if HVPG was measured no longer than 48 hours after the treatment (6). Additionally, NSBBs are currently recommended for a majority of patients with cirrhosis. It has been reported that patients whose HVPG lowered significantly after receiving NSBBs (hemodynamic responders) have lower bleeding rate and mortality compared to non-responders (18,20-22). The marked effect of ameliorating prognosis of administrating NSBBs is largely ascribed to its potential to lower HVPG (22-24). However, under circumstances that NSBBs were administrated before HVPG measurement, the HVPG lowered by medication may fail to reflect the real severity level of cirrhosis and whether the patient responds well to medication, and thus introduce bias in judgement. Besides, it has been reported that hemodynamic responders at baseline could be not continued to respond after a certain period, and the actual prognosis is significantly worse for these patients than that predicted by baseline-HVPG (25,26).
One intuitive attempt to improve the predictive effect of HVPG in these patients is to perform HVPG measurement routinely during follow-up. However, due to its invasiveness, extra expenses and uncertain benefit to patients, routine HVPG measurement is not recommended currently and is still under debate (27,28). Nevertheless, although limited, recent studies have provided evidence supporting that an increased predictive accuracy may be achieved via additional measurement of HVPG during follow-up. As reported by Sanyal et al. in patients with liver fibrosis, HVPG might change over time and taking HVPG changes measured at week 48 and 96 after baseline into consideration significantly increased the prognostic performance of HVPG for clinical events including ascites, hepatic encephalopathy and variceal hemorrhage (29). Similarly, results of another recent study showed that in hepatic C virus infected patients receiving interferon-free therapy, combining HVPG measured after and before therapy could provide more decompensation-prognostic efficacy compared to a single HVPG before the therapy (30). Our study suggested that a single baseline-HVPG measurement was insufficient for predicting rebleeding in patients with cirrhosis who received secondary prevention of variceal rebleeding. Thus, a longitudinal assessment of HVPG might be needed. However, repeated HVPG measurements are not routine clinical practice in most of the centers, urging the need of development of non-invasive tools as surrogate measurements of HVPG.
Our study has several limitations. First, this study is a retrospective study including patients from a single center with limited sample size, bringing possible bias. The conclusion of this study may need validation in a prospective cohort with adequate sample size. Besides, all the patients did not receive NSBB, which may bring heterogeneity, somehow compromising our conclusion.
In summary, we found that in patients with cirrhosis receiving secondary prevention, a trend of decreasing AUC by time was observed when using baseline HVPG to predict rebleeding. Besides, a decreasing trend of HR of rebleeding by time was found in patients with stratified HVPG category. Taking together, these findings suggested that timing could weaken the predictive value of HVPG for rebleeding; and a single baseline-HVPG measurement has only limited rebleeding predictive performance in patients with cirrhosis who receive secondary prevention of variceal rebleeding.
Acknowledgments
Funding: This study has been supported by the grants from National Natural Science Foundation of China (81600510; 81770606); Guangdong Science Fund for Distinguished Young Scholars (2018B030306019); Guangzhou Industry-Academia-Research Collaborative Innovation Major Project (201704020015).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare related to this article.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Medical Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University (No. 2019-51) and the patient informed consent was achieved.
References
- Qi X, Berzigotti A, Cardenas A, et al. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol 2018;3:708-19. [Crossref] [PubMed]
- Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic Venous Pressure Gradient Predicts Clinical Decompensation in Patients With Compensated Cirrhosis. Gastroenterology 2007;133:481-8. [Crossref] [PubMed]
- Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999;117:626-31. [Crossref] [PubMed]
- Blendis L, Wong F. The hyperdynamic circulation in cirrhosis : an overview. Pharmacol Ther 2001;89:221-31. [Crossref] [PubMed]
- Bosch J, García-pagán JC. Prevention of variceal rebleeding. N Engl J Med 2003;361:952-4. [PubMed]
- Avgerinos A, Armonis A, Stefanidis G, et al. Sustained rise of portal pressure after sclerotherapy, but not band ligation, in acute variceal bleeding in cirrhosis. Hepatology 2004;39:1623-30. [Crossref] [PubMed]
- Merkel C, Bolognesi M, Bellon S, et al. Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal varices. Gastroenterology 1992;102:973-9. [Crossref] [PubMed]
- Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in cirrhosis. N Engl J Med 2005;353:2254-61. [Crossref] [PubMed]
- Garcia-Tsao G, Groszmann RJ, Fisher RL, et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5:419-24. [Crossref] [PubMed]
- Berzigotti A, Rossi V, Tiani C, et al. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J Gastroenterol 2011;46:687-95. [Crossref] [PubMed]
- Li G, Yuan H, Li X, et al. Hepatic venous pressure gradient is a useful predictor in guiding treatment on prevention of variceal rebleeding in cirrhosis. Int J Clin Exp Med 2015;8:19709-16. [PubMed]
- Monescillo A, Martinez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology 2004;40:793-801. [Crossref] [PubMed]
- Silva-Junior G, Baiges A, Turon F, et al. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology 2015;62:1584-92. [Crossref] [PubMed]
- Ready JB, Robertson AD, Goff JS, et al. Assessment of the risk of bleeding from esophageal varices by continuous monitoring of portal pressure. Gastroenterology 1991;100:1403-10. [Crossref] [PubMed]
- Abraldes JG, Villanueva C, Bañares R, et al. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008;48:229-36. [Crossref] [PubMed]
- Zhao JR, Wang GC, Hu JH, et al. Risk factors for early rebleeding and mortality in acute variceal hemorrhage. World J Gastroenterol 2014;20:17941-8. [Crossref] [PubMed]
- D’Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: A 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther 2014;39:1180-93. [Crossref] [PubMed]
- Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990;99:1401-7. [Crossref] [PubMed]
- Sauerbruch T, Mengel M, Dollinger M, et al. Prevention of Rebleeding From Esophageal Varices in Patients With Cirrhosis Receiving Small-Diameter Stents Versus Hemodynamically Controlled Medical Therapy. Gastroenterology 2015;149:660-8.e1. [Crossref] [PubMed]
- Villanueva C, Aracil C, Colomo A, et al. Acute Hemodynamic Response to β-Blockers and Prediction of Long-term Outcome in Primary Prophylaxis of Variceal Bleeding. Gastroenterology 2009;137:119-28. [Crossref] [PubMed]
- Villanueva C, Minana J, Ortiz J, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med 2001;345:647-55. [Crossref] [PubMed]
- Turco L, Villanueva C, La Mura V, et al. Lowering Portal Pressure Improves Outcomes of Patients With Cirrhosis, With or Without Ascites: A Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:313-27.e6. [Crossref] [PubMed]
- Kim SG, Kim TY, Sohn JH, et al. A Randomized, Multi-Center, Open-Label Study to Evaluate the Efficacy of Carvedilol vs. Propranolol to Reduce Portal Pressure in Patients with Liver Cirrhosis. Am J Gastroenterol 2016;111:1582-90. [Crossref] [PubMed]
- Schwarzer R, Kivaranovic D, Paternostro R, et al. Carvedilol for reducing portal pressure in primary prophylaxis of variceal bleeding: a dose-response study. Aliment Pharmacol Ther 2018;47:1162-9. [Crossref] [PubMed]
- Merkel C, Bolognesi M, Berzigotti A, et al. Clinical significance of worsening portal hypertension during long-term medical treatment in patients with cirrhosis who had been classified as early good-responders on haemodynamic criteria. J Hepatol 2010;52:45-53. [Crossref] [PubMed]
- Augustin S, González A, Badia L, et al. Long-term follow-up of hemodynamic responders to pharmacological therapy after variceal bleeding. Hepatology 2012;56:706-14. [Crossref] [PubMed]
- Ulrich T, Lia B, Claudio P, et al. Should we routinely measure portal pressure in patients with cirrhosis, using hepatic venous pressure gradient (HVPG) as guidance for prophylaxis and treatment of bleeding and re-bleeding? No. Eur J Intern Med 2011;22:5-7. [Crossref] [PubMed]
- Merkel C, Montagnese S. Should we routinely measure portal pressure in patients with cirrhosis, using hepatic venous pressure gradient (HVPG) as guidance for prophylaxis and treatment of bleeding and re-bleeding? Yes! Eur J Intern Med 2011;22:1-4. [Crossref] [PubMed]
- Sanyal AJ, Harrison SA, Ratziu V, et al. The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials. Hepatology 2019;70:1913-27. [Crossref] [PubMed]
- Mandorfer M, Kozbial K, Schwabl P, et al. Changes in Hepatic Venous Pressure Gradient Predict Hepatic Decompensation in Patients Who Achieved Sustained Virologic Response to Interferon-Free Therapy. Hepatology 2019. [Epub ahead of print]. [Crossref] [PubMed]


