
Growth hormone promotes human endometrial glandular cells proliferation and motion through the GHR-STAT3/5 pathway
Introduction
Endometrium dysfunction, including intrauterine adhesion (IUA), is reported to contribute to more than half of implantation failures in assisted reproductive technology (ART) (1). IUAs usually occur following invasive intrauterine procedure, which injures the endometrium/myometrium (2). As one of the prevalent endometrial lesion diseases, IUA plays a critical role in secondary infertility at reproductive age, and seriously threatens the fertility requirements and life quality of women (3). Currently, the standard therapy of IUA is transcervical resection of adhesion (TCRA), and the intrauterine device placement, antiadhesive barrier gels, intrauterine balloon, or high-dose estrogen are the adjuvant treatment methods (4). However, therapies are still limited to meet the challenge of different types of IUA, for example, estrogen administration failed to preventively against IUA in some studies (5), and almost 62.5% of the patients with severe IUA relapse after hysteroscopy (6). Further research is needed to establish the therapies to promote the structure and functional integrity in various IUAs, especially severe IUA, with difficulty in endometrium regeneration.
As a regulator of growth and development, growth hormone (GH) could be a promising drug in IUA treatment. Pituitary somatotrophs secrete GH under the positive regulation of GH-relating hormone and the negative regulation of somatostatin and Janus kinase 1 (JAK1). Growth hormone receptor (GHR) binding of GH leads to transcription of target genes, such as insulin-like growth factor 1 (IGF1) and IGF2, via JAK2 or ERK pathway to promote cell proliferation and tissue growth (7). The roles of GH in female reproduction is recently reported, which including sex differentiation and maturation, ovary maturation and ovulation, pregnancy and fetal growth, and pubertal mammary development and lactation (8). For example, GH facilitates folliculogenesis and gametogenesis, which is associated with its steroidogenesis action and interaction with IGF (8). During recent three decades, GH has been reported to promote endometrial thickness and to achieve the desired therapeutic effect in frozen-thawed embryo transfer of patients with IUA with the combined administration of estrogen (9). However, the mechanism behind the optimistic influence of GH is far from being clear.
Therefore, this study aims at testing whether GH improves human endometrial glandular cells (hEGCs) proliferation through the GHR-STAT signaling pathway.
Methods
Isolation and culture of hEGCs
All procedures were conducted following a protocol approved by the Ethics Committee of the Third Xiangya Hospital of Central South University. The protocol of isolation and culture of hEGCs were according to the previous report, which demonstrated the method could generate over 95% vimentin-positive and cytokeratin-7 negative hEGCs (10). Briefly, the endometrial biopsies were washed twice with PBS containing Penicillin-Streptomycin (Pen/Strep, 1 nM 1x working solution), and gently cut into 1 mm3 piece using forceps and scalpels. The tissue pieces were transferred to a 15 mL Falcon tube containing 0.1% Collagenase IV and incubated for 1 h at 37 °C. Pipette the digested matter into a 150 µm cell filter, and the filtrate was then passed a 40 µm cell strainer to collect the residue by PBS washing. Spin at 1,000 rpm for 5 min to remove the supernatant and resuspend the single-cell suspension in 10% FBS DMEM/F12 media then plated directly onto cell culture Petri dishes. Incubate the petri dish at 37 °C and 5% CO2 and renew the media every three days.
Cell proliferation analysis
The hEGCs were isolated and cultured as the previous description, then exposed to GH at 0, 50, 100, 200 ng/mL or AG490 at 50nM, according to the design, for 24 h till cell viability analysis. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide) cell proliferation assay were performed then. MTT reagent (5 mg/mL) was added to each well and incubated at 37 °C for four h. Next, 150 µL of MTT detergent reagent was added to each well and incubated in the dark at room temperature for two h. Absorbance reads were measured at 490 nm by a microplate reader (Bio-Tek). For EdU proliferation assays, the hEGCs were stained for EdU labeling in 1:1,000 dilution for 12 h, fixed in 10% formalin for fifteen minutes, washed and stained with 488-Azide Fluorophore for thirty minutes in the dark. The cells were then washed and sorted through Fluorescence-activated cell sorting (FACS) and analyzed for % live EdU positive cells. EdU assay was performed using Click-iT EdU Alexa Fluor 488 Imaging Kit (Invitrogen, C10639).
Cell migration capability assay
For cell stretch experiments, the hEGCs were seeded in 6-well plated with 5×105 cells/well and cultured in complete medium for 2–3 days, until fully confluent. A sterile 1ml pipette tip was used to create a linear scratch injury across the cell monolayer, then the cells were rinsed in serum-free medium and then processed for microscope inspection at 0, 24 and 48 h, separately. Transwell invasion assays were performed using Transwell chambers (24-well, 8 µm pore size) dependent on Matrigel-coated membranes. The upper Transwell chambers contained culture medium without FBS while the lower was filled with culture media containing 10% FBS. The Transwell chambers were incubated at 37 °C for one day. The lower Transwell chambers were added paraformaldehyde to fix the invaded cells, which they were stained with 1% crystal violet. Cell counting was performed under a microscope.
Cell cycle analysis
Flow cytometry analysis was used to perform cell cycle analysis. The isolated hEGCs were exposed to different dose of GH or AG490 for 24 h according to the design. Cells were washed with PBS, harvested, and fixed with 70% ethanol for 12 h at 4 °C. The hEGCs were stained in PBS containing 150 µg/mL propidium iodide (PI) at 37 °C for 30 min in the dark, following centrifugation, washing, and resuspension. Cytomics FC 500 flow cytometry (Beckman Coulter, Webster, Texas, USA) was used for fluorescence detection and analyzation.
Immunocytochemistry (ICC)
The hEGCs were seeded in 6-well plate, which were already implanted polylysine-coated slides, with 5×105 cells/well and cultured in complete medium for 2 days. The cells on the slides were fixed in 10% formalin for thirty minutes at 37 °C, washed and permeabilized in 1% Triton X-100 at 37 °C for thirty minutes. 1% bovine serum albumin (BSA, HyClone, USA) were used to block non-specific binding of antibodies. For hEGCs identification, keratin and vimentin antibody were incubated with slides for 12 hours, the anti-GHR antibody was used to detect GHRs, and mouse IgG as isotype. The slides were incubated with peroxidase-conjugated goat anti-mouse IgG antibody following washing. Then, the slides were washed, overlaid with 3,3-diaminobenzidine (DAB), and counterstained with hematoxylin. The slides were then observed using an Olympus microscope.
Gene expression analysis
The expressions of STAT3 and STAT5 previous study (11). Briefly, cells were extracted using TRIZOL reagent (Invitrogen, USA) and cDNAs were synthesized by PrimeScript RT reagent Kit with gDNA Eraser, according to the manufacturer’s recommendation. RT-PCR was performed by Applied Biosystems 7900HT Fast Real-Time PCR System (Thermo, USA). Primers used in this study were: STAT3 (F-AGCAAAGAATCACATGCCACT, R-CCATTGGCTTCTCAAGATACCTG, STAT5 (F-ACCCAGCGCAGGCAA, R-TTCTCCTTGGAGCTGCTGAG), and beta-actin (F-ACCCTGAAGTACCCCATCGAG, R-AGCACAGCCTGGATAGCAAC). The relative gene expression was represented by the ratio of the target gene to the control gene using the formula 2-(∆∆Ct), where ∆∆Ct= (CtTarget-Cthouse-keeping genes)treatment - (CtTarget-Cthouse-keeping genes)control. Normalization of relative gene expression was expressed as a ratio to the expression of the controls (12).
Western blotting analysis
The related protein expression was analyzed by western blot. The cells were exposed to GH at 0, 20, 100, 200 ng/mL or AG490 at 50 nM, according to the design, until 75% confluent. The cells were harvested and extract protein with RIPA lysis buffer with PMSF. 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separate microliter protein lysate, and PVDF membranes were used for protein transmembrane. Then, the membranes were incubated with 5% bull serum albumin for 90 min, washed, and incubated in the primary antibodies overnight at 4 °C. Primary antibodies dilutions were performed as following: STAT3 (1:1,000), p-STAT3 (1:2,000), STAT5 (1:500), p-STST5 (1:1,000), and actin (1:5,000). The PVDF membrane incubated with secondary antibody for 90 min at room temperature. The signals were visualized following incubation with enhanced chemiluminescence. The blotting bands were quantified with quantity one software.
Statistical analysis
Prism GraphPad software version 8 (GraphPad Software, Inc., La Jolla, CA) was used to perform statistical analysis and generate figures. One-way ANOVA was used to analyze the data among different GH-treating groups. Moreover, unpair t-test was used to analyze the data between Con vs. GH groups and GH vs. GH + AG490 groups. The results were presented as mean ± SEM. P<0.05 indicated a significant difference.
Results
GH promoted proliferation, activated cell cycle, and migration capability of hEGCs
The isolated hEGCs were exposed to different dose of GH. MTT assay showed that GH at the dose of 100 and 200 ng/mL significantly promoted hEGCs viability (Figure 1A). Similarly, GH in a concentration of 100 and 200 ng/mL remarkably improved hEGCs proliferation in EdU assay (Figure 1B,C).
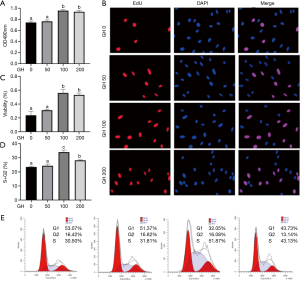
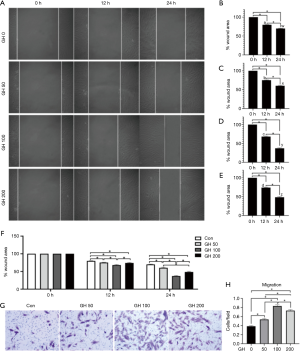
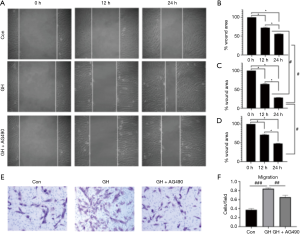
Moreover, GH significantly increased the percentage of cells in G2 or S phases and reduced of those in G1 phase (Figure 1D,E). However, not any difference was observed between 0 and 50 ng/mL GH groups in all the above tests (P>0.05). The migration of hEGCs was analyzed by scratch wound assay and Transwell assay, and the results were shown as Figure 2. In scratch wound assay, the results indicated that GH promoted the motility of hEGCs, 100 ng/mL GH in the media had optimum efficiency (Figure 2A,B,C,D,E,F). Moreover, the results were similar, as shown in the Transwell assay (Figure 2G,H).
GH activated GHRs expression in hEGCs
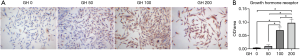
The expression of GHRs in hEGCs was detected by ICC after the hEGCs exposed to GH in different concentration for 24 hours. The results demonstrated that the expression of GHRs was enhanced after GH treatment and exhibited dose-dependent changes (Figure 3A,B). GH at a concentration of 100 ng/mL was most efficient than those of others. Therefore, the GH dosage of 100 ng/mL was chosen for the next research.
GHR inhibitor suppressed the proliferation and migration induced by GH in hEGCs
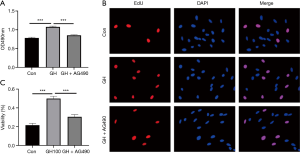
MTT assay and EdU assay were performed after GH exposed for 24 hours. The results showed GH promoted the viability of hEGCs both in MTT assay (Figure 4A) and EdU assay (Figure 4B,C), and AG490, a GHR inhibitor, blocked the proliferative effect of GH (Figure 5). Cell migration tests were processed following the proliferation assay (Figure 5). Moreover, AG490 reversed hEGCs migration induced by GH in the scratch assay (Figure 5A,B,C,D) and Transwell assay (Figure 5E,F), indicated GH enhanced the motion of hEGCs was GHR-dependent.
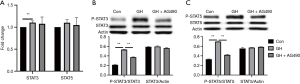
GH activated STAT3 expression in hEGCs, which could be reversed by GHR inhibitor
GH supplementation in the media increased the expression of STAT3 on mRNA level (Figure 6A), but not on protein level (Figure 6B). GH neither affect mRNA (Figure 6A) nor protein (Figure 6C) expression of STAT5 in hEGCs. GHR inhibitor also had a negligible effect on the expression of STAT3 and STAT5 (Figure 6). GH activated phosphorylation of STAT3 and 5, which was reversed by GHR inhibitor (Figure 6B,C).
Discussion
IUA is one of the most common reproductive system diseases in women of childbearing age. Clinically, TCRA is the standard therapy, and high-dose estrogen is the adjuvant treatment methods for IUA treatments (4). However, estrogen therapy presents notable barriers, and the therapy of severe IUA still meets the challenge of endometrial regeneration in the scar tissue of the uterus. GH, serve as adjuvant treatment empirically in assisted reproduction, promotes pregnancy rate, and upregulates GH-dependent signaling pathway in RL95-2 cell line (13). Thus, this study aims at exploring the effect of GH on the hEGCs and preliminary discussing on the mechanism. The results show that GH promotes the proliferation, motion, and actives the cell cycle and GRHs of hEGCs. However, those effects of GH could be reversed by AG490, a GHR inhibitor. GH administration does not affect the abundance of STAT3 and STAT5 protein; however, GH actives that two protein in the absence of GHR inhibitor.
GH is important in growth and development for its role in growth, cell reproduction, and regeneration. It was observed the expression of GH, both in gene and protein level, in the human ovary, revealing that GH modulates the intracellular GH related signaling pathway in a paracrine action (14). Administration with GH in the treatment of primary infertility women with panhypopituitarism promoted endometrial development and ovarian response (15). In another report, GH has demonstrated to augment the endometrium thickness and promoted human RL95-2 cell viability (13). Similarly, we found that GH promoted the proliferation, migration, and cell cycle of isolated hEGCs. Thus, GH increases the endometrium thickness may relate to the proliferative effect of GH to endometrial cells.
GH actives the intracellular signaling, such as the JAK-STAT pathway, by binding its cell surface receptor, GHRs. GHRs was also found to express in the endometrium of human and animal, such as cows, rat, and fish (16-18). GHR deficiency mice show a delay in puberty, decrease in preovulatory follicles, and damage to reproduction (19,20). These reports indicated the potential role of GHRs in ovary development. Our results revealed that GH treatment active GHRs and GHR inhibitor suppressed the promotive effects of GH in proliferation and motion of hEGCs, which indicated the effects of GH on hEGCs is GHR-dependent. JAK2-STAT3/5 was an important downstream pathway of GHRs to play regulator role of GH in metabolism. Wang et al. processed a comparative analysis of the endometrial tissue expression profiles of pigs on days 9, 12, 15, and JAK-STAT pathway was enriched in differentially expressed genes (21). In the episode of decidualization of the endometrium, STAT3 is among the most down-regulated genes (22,23). Overexpression of protein inhibitor of activated STAT 3, an inhibitor of STAT3, attenuated the phosphorylation of STAT3 and suppressed the growth of HO-3687 cell lines (24).
Moreover, AG490 reverses JAK2-STAT5 pathway activation mediated by GH in human endometrial cells (13). Reasonably, STAT3/STAT5, activated by GHRs, play the roles in endometrial cell proliferation and endometrial development, which agrees with the results in this study. However, further studies are needed to explore the effects of other signaling pathways, such as phosphatidylinositol three kinase-protein kinase B or mitogen-activated protein kinase, mediated by GHRs on hEGCs.
Collectively, GH supplementation promoted isolated hEGCs proliferation and motion, which is GHR-JAK-STAT3/5 signaling pathway-dependent. This provides evidence for GH-GHR-STAT3/5 axis in the hEGCs growth and IUA treatment.
Acknowledgments
Funding: This study is supported by the Natural Science Foundation of China (Grant No. 81671492). The study received approval from the institutional review board of the Third Xiangya Hospital of Central South University (September 4, 2019; number: 2019-S456). Bingsi Gao is supported by China Scholarship Council, file number 201806370178.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The need for ethics approval and consent of the present study was waived by Institutional Review Board of Third Xiangya Hospital of Central South University. Written informed consent was obtained from all patients.
References
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006;12:731-46. [Crossref] [PubMed]
- Gilman Barber AR, Rhone SA, Fluker MR. Curettage and Asherman's syndrome-lessons to (re-) learn? J Obstet Gynaecol Can 2014;36:997-1001. [Crossref] [PubMed]
- Laganà AS, Garzon S, Franchi M, et al. Translational animal models for endometriosis research: a long and windy road. Ann Transl Med 2018;6:431. [Crossref] [PubMed]
- Ludwin A, Martins WP, Ludwin I. Ultrasound-guided sequential intrauterine balloon dilatation for the prevention of adhesions: Intrauterine balloon dilatation. Ultrasound Obstet Gynecol 2019;54:566-8. [Crossref] [PubMed]
- Healy MWD, Schexnayder BM, Connell MTD, et al. Intrauterine adhesion prevention after hysteroscopy: a systematic review and meta-analysis. Am J Obstet Gynecol 2016;215:267-75. [Crossref] [PubMed]
- Szymański R, Kamiński P, Marianowski L. Electroresectoscopy in submucous fibroids, intrauterine adhesions and uterine malformation treatment. Ginekol Pol 2000;71:1031-5. [PubMed]
- Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol 2018;14:285-300. [Crossref] [PubMed]
- Ma H, Shi X, Fu Z, et al. Potential roles of microRNA in levonorgestrel-treated uterine leiomyoma cells. Transl Cancer Res 2018;7:1376-83. [Crossref]
- Yang JY, Li H, Lu N, et al. Influence of growth hormone supplementation in patients with thin endometrium undergoing frozen embryo transfer. Reproductive and Developmental Medicine 2019;3:49-53. [Crossref]
- Lei S, Cao Y, Sun J, et al. H 2 S promotes proliferation of endometrial stromal cells via activating the NF-κB pathway in endometriosis. Am J Transl Res 2018;10:4247. [PubMed]
- Chen S, Wang M, Yin L, et al. Effects of dietary tryptophan supplementation in the acetic acid-induced colitis mouse model. Food Funct 2018;9:4143-52. [Crossref] [PubMed]
- Chen S, Tan B, Xia Y, et al. Effects of dietary gamma-aminobutyric acid supplementation on the intestinal functions in weaning piglets. Food Funct 2019;10:366-78. [Crossref] [PubMed]
- Cui N, Li AM, Luo ZY, et al. Effects of growth hormone on pregnancy rates of patients with thin endometrium. J Endocrinol Invest 2019;42:27-35. [Crossref] [PubMed]
- Schwarzler P, Untergasser G, Hermann M, et al. Selective growth hormone/placental lactogen gene transcription and hormone production in pre- and postmenopausal human ovaries. J Clin Endocrinol Metab 1997;82:3337-41. [PubMed]
- Drakopoulos P, Pluchino N, Bischof P, et al. Effect of Growth Hormone on Endometrial Thickness and Fertility Outcome in the Treatment of Women with Panhypopituitarism: A Case Report. J Reprod Med 2016;61:78-82. [PubMed]
- Chen J, Cao M, Zhang A, et al. Growth Hormone Overexpression Disrupts Reproductive Status Through Actions on Leptin. Front Endocrinol (Lausanne) 2018;9:131. [Crossref] [PubMed]
- Guggeri D, Meikle A, Carriquiry M, et al. Long-term effect of early nutrition on endocrine parameters and liver and endometrial gene expression of the members of the somatotrophic axis in Hereford heifers. Reprod Domest Anim 2018;53:930-6. [Crossref] [PubMed]
- Chen Y, Liu F, Nong Y, et al. Clinical efficacy and mechanism of growth hormone action in patients experiencing repeat implantation failure. Can J Physiol Pharmacol 2018;96:929-32. [Crossref] [PubMed]
- List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev 2011;32:356-86. [Crossref] [PubMed]
- Sosa C, Carriquiry M, Chalar C, et al. Endometrial expression of leptin receptor and members of the growth hormone-Insulin-like growth factor system throughout the estrous cycle in heifers. Anim Reprod Sci 2010;122:208-14. [Crossref] [PubMed]
- Wang Y, Xue S, Liu X, et al. Analyses of Long Non-Coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium. Sci Rep 2016;6:20238. [Crossref] [PubMed]
- Lira-Albarran S, Durand M, Barrera D, et al. A single preovulatory administration of ulipristal acetate affects the decidualization process of the human endometrium during the receptive period of the menstrual cycle. Mol Cell Endocrinol 2018;476:70-8. [Crossref] [PubMed]
- Lira-Albarran S, Durand M, Larrea-Schiavon MF, et al. Ulipristal acetate administration at mid-cycle changes gene expression profiling of endometrial biopsies taken during the receptive period of the human menstrual cycle. Mol Cell Endocrinol 2017;447:1-11. [Crossref] [PubMed]
- El Khoury D, Matar R, Touma T. Curcumin and endometrial carcinoma: an old spice as a novel agent. Int J Womens Health 2019;11:249-56. [Crossref] [PubMed]


