Barley β-glucan in poultry diets
Introduction
Barley is most commonly grown for the production of beer and malt whiskey. It is also used as a feed ingredient in animal diets, especially in Europe. Barley is considered a high fiber (~22%) cereal and is thus low in protein (10-12% crude protein). Hull-less barley is lower in fiber and is the type more commonly used in poultry feeds.
For years the use of barley in poultry diets was limited due to its negative effects on bird performance. This poor performance was found to be due to the β-glucans in barley (see Table 1). When barley-based diets were supplemented with exogenous enzymes there was an improvement in both growth and feed efficiency. When barley β-glucans were added to a corn-based diet there was an increase in the viscosity of the intestinal contents of the chicks fed. When exogenous enzymes were added to the corn-based diets with barley β-glucans the intestinal viscosity was returned to near control levels. The researchers concluded that the β-glucans of barley are the cause of poor chick performance, most likely due to the increase in the viscosity of the intestinal contents (1). The negative effects related to the use of barley in poultry diets was extensively reviewed (2) and is beyond the scope of this article (see Table 1).

Full table
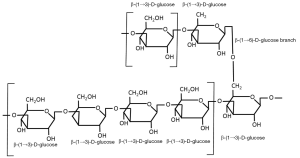
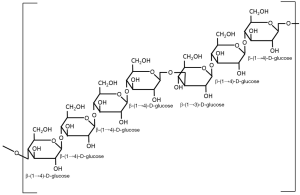
Beta-glucans are structural components of cell walls in many different sources including bacteria, fungi, algae and yeast. They are also found in grains such as barley, oats and rye. The structure of the β-glucans, however, vary among the different sources and account for the differences in their physiological function. Beta-glucans consist of β-ᴅ-glucose molecules joined by (1→3)- or (1→4)-glycosidic bonds. The structure of the chain depends on the relative number of (1→3)- and (1→4)-glycosidic bonds between the repeating glucose units. Beta-glucans from yeast and fungi consist of a backbone of glycopyranosyl molecules joined by (1→3)-β-links. From this backbone are side chains joined with (1→6)-β-links (see Figure 1). This gives the (1→3)(1→6)-β-glucans of fungi and yeast a branched structure. Fungal β-glucans have short branches while they are long in yeast. Bacterial β-glucans, on the other hand, are unbranched with only (1→3)-β-linkages between the glycopyranosyl molecules. In barley, as with most cereal grains, the β-glucans are not branched. Barley β-glucans have (1→4)-β-links between the glycopyranosyl molecules (see Figure 2). The linked segments are considered semi-flexible portions of the chain and the (1→3)-bonds introduce kinks to that chain (3). So yeast and fungi have (1→3)(1→6)-β-glucans while cereal grains have (1→3)(1→4)-β-glucans and bacteria have (1→3)-β-glucans. These differences are reflected in differences in their functionality.
Botanical polysaccharides are gaining increased interest in biomedical research due to their broad spectrum of therapeutic properties, together with relatively low toxicity. Beta-glucans have also been studied as an alternative to antibiotics in food animal production, including poultry. With the increased pressure to reduce antibiotic use in food animal production, producers are looking at boosting immune function as an alternative approach to reducing the detrimental effects of harmful intestinal pathogens.
Before one can understand the function of β-glucans in poultry diets one must first understand the avian immune system and the similarities and differences to the mammalian system.
Avian immune system
The avian immune system differs from that of mammals sufficiently that mammalian models cannot necessarily be extrapolated to birds. While the avian system shares many similarities with mammalian systems, there are differences in the genes and molecules involved, the cells and organs involved, as well as the functional mechanisms. Chickens, for example, have a different selection of Toll-like receptors (TLRs), defensins, chemokines, antibodies and other immune molecules. Birds do not have eosinophils although the functional equivalent to the mammalian neutrophil is the avian heterophil. Birds do not have lymph nodes, but do have a Bursa of Fabricius, which mammals do not. The Bursa of Fabricius is important in the development of B-cell receptors. The mechanisms by which the different receptors are generated are also fundamentally different.
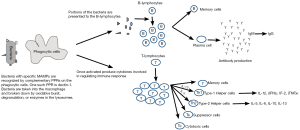
The avian immune system has two interactive components, the innate and the adaptive immune systems. The innate component is basically the first line of defense against any potential pathogens and has an important role in triggering the more complex adaptive immune system (see Figure 3).
The innate immune system includes the physical and chemical barriers the body has for keeping pathogens out. These barriers include the skin, mucosal secretions, and gastric juices. The innate immune component also includes blood cells which act to destroy pathogens. The phagocytic cells of the blood system which engulf and destroy pathogens include macrophages, heterophils (the avian equivalent to mammalian neutrophils), thrombocytes, and natural killer cells. These different cell types are usually triggered by microbe-associated molecular patterns (MAMPs—sometimes referred to as pathogen-associated molecular patterns, or PAMPs) which are recognized by pattern recognition receptors (PPRs) on the surface of the immune cells. An example of a MAMP would be the lipopolysaccharides in the cell wall of gram-negative bacteria and lipoteichoic acid in the cell wall of gram-positive bacteria. Adaptive immunity is much more complex than innate immunity. The adaptive immune system is further divided into two portions—humoral and cell-mediated immunity. The humoral response involves the production of antibodies by B-lymphocytes. The cell-mediated response involves attacks of infected cells by cytotoxic and helper T-lymphocytes.
The two components of the immune system, innate and adaptive, are interactive. After leukocytes of the innate immune system are activated, they produce cytokines and present the antigens to the T- and B-lymphocytes of the adaptive immune system. While the innate system is rapid and non-specific, the adaptive system is slower and highly specific. Because of memory cells, however, the adaptive response speed is increased with subsequent contacts with a particular antigen.
Heterophils are the avian equivalent of mammalian neutrophils. They are polymorphonuclear and actively phagocytose invading pathogens. They are the first cell type to respond in numbers to an induced innate immune response (4). After the bacteria have been brought into the heterophils in a phagosome they are killed by a variety of mechanisms. One such mechanism is referred to as the ‘respiratory’ or ‘oxidative’ burst. NADPH oxidase is activated in the heterophils and produces large quantities of superoxide, which is then converted to hydrogen peroxide and finally to hydrochlorous acid. The hydrochlorous acid is thought to have the bactericidal activity of the heterophils (4). Heterophils also kill bacteria with the release of proteins in granules into the phagosome. This process is referred to as degranulation. These protein molecules include defensins.
Defensins are anti-microbial peptides found in plants, insects, birds and mammals and have two main functions. The first is to bind microbial cell membranes and form pores leading to the death of the microbe. The second function of defensins is to chemo-attract the cells of the innate immune system with roles in microbial attack including the macrophages and mast cells (5). Defensins are species-specific. In mammals there are three classes of defensins including α-, β- and ϴ-defensins. In birds, however, there are only β-defensins (6). Beta-defensins are expressed primarily in the leukocytes and epithelial cells. Defensins are either constitutively expressed in granules of phagocytic cells or are inducible in response to signaling from PRRs (4).
Macrophages are crucial players in both the innate and adaptive immune responses. Macrophages originate from bone marrow stem cells. They start out as monocytes which enter the blood stream and are a major phagocytic component in avian blood. Monocytes can develop into macrophages which are present in a variety of body tissues. Tissue macrophages are typically identified by their tissue location. For example, those in the lungs are alveolar macrophages; in bone, osteoblasts, in the brain, microglia cells; and histiocytes in the connective tissue. The production is genetically controlled with broiler chickens producing significantly lower numbers of macrophage-type colonies than White Leghorn chickens (7).
The bacteria or other antigens bind to the surface of the macrophage. The macrophages then bring them into their cytoplasm in the form of a phagosome. One macrophage can internalize several bacteria. The phagosome then fuses with a lysosome so that the enzymes present in the lysosome can be used to degrade the internalized bacteria. Macrophages then present bacterial peptides or other antigenic determinants to immune cells like the B- or T-lymphocytes to initiate the adaptive immune response (8). Chicken macrophages also produce several types of cytokines. Cytokines, which are signaling proteins, are important in regulating the immune response. Cytokines are produced by a variety of different cell types and the types of cytokines produced determine what effect they will have. For example, interleukin (IL)-12 is produced by activated macrophages and stimulates type 1 helper T-lymphocytes (Th1). This leads to the production of additional cytokines which play important roles in cell-mediated immunity such as IL-1β, interferon (IFN)γ, IL-2 and tumor necrosis fact (TNF)α. The production of IL-4 results in the development of type 2 helper T-lymphocytes (Th2) resulting in the production of IL-5, IL-6, IL-10 and IL-13 which are involved in the humoral immune response.
In mammals, B-cells and plasma cells secrete IgM in the primary immune response and switch to IgG for the secondary immune response. This differentiation is much smaller in chickens. IgM has five subunits joined in a radial fashion similar to a star. Each subunit has two antigen-binding sites so that the IgM molecule can potentially bind ten antigen molecules. After production of IgM, the B-cells switch to the production of IgG which are made up of a single Y-shaped molecule with two different antigen binding sites. B-cells and plasma cells in the intestinal lymphoid tissues secrete a third immunoglobulin, IgA which is secreted into the digestive, ocular, genital and respiratory tracts to provide protection by preventing potential pathogens from adhering. IgA typically has two joined subunits. T-cells are involved in regulating the cell-mediated immune responses. There are many types including T-effector cells, T-helpers and T-suppressors. T-cells also function as memory cells in the same manner as B memory cells (9).
Effect of β-glucans on the immune system
Evidence is accumulating to show that the composition of the diet influences immune system function. As a result, a number of different components of food have been studied in this regard. Beta-glucans are one of these food components.
Beta-glucans from fungi and yeast function as MAMPs and trigger an immune response. The cell types involved in the innate immune system include the leukocytes heterophils, monocytes/macrophages, and natural killer cells. These cells serve several functions. They are involved with phagocytosis of pathogens, free radical production (oxidative burst), cytokine production, and antigen presentation to lymphocytes of the adaptive immune system. The molecular weights and degree of branching will affect the immune response of β-glucans in regards to cytokine production (10-12), enhancement of phagocytic activities and the level of bactericidal activity (13-15).
In vitro studies have shown that (1→3)(1→6)-β-glucans can enhance the functional activity of macrophages as well as activate antimicrobial activity of mononuclear cells and neutrophils (16,17). This enhanced immune function is the result of increased pro-inflammatory cytokine production (18), oxidative burst and chemokine production (16).
The role of β-glucans as biologically active immunomodulators in mammalian systems has been well documented. With the desire to find alternatives to antibiotics in poultry production, research looking at the effect of β-glucans on the avian immune system has increased recently.
As with mammals, exposure to yeast β-glucans has been shown to enhance proliferation and phagocytizing efficiency of avian macrophages (19) and heterophils (20). Inclusion of purified yeast β-glucan was shown to stimulate phagocytosis, bactericidal killing and oxidative burst in chickens (20). Broiler chickens fed diets supplemented with yeast β-glucan [a (1→3)(1→6)-β-glucan] had enhanced humoral (14,19) and cell-mediated immune responses (21,22). (1→3)(1→6)-β-glucan has been shown to significantly enhance macrophage phagocytic function in chickens fed diets containing as little as 20 mg/kg of (1→3)(1→6)-β-glucan when compared to chickens fed a basal diet (8).
In addition, the immune-enhancing effects of (1→3)(1→6)-β-glucans have also been shown to be effective in preventing colonization by several economically important pathogens, including Salmonella enterica and Escherichia coli (20,23). Dietary (1→3)(1→6)-β-glucan supplementation has been shown to prevent Salmonella Enteritidis colonization in the visceral organs of layer pullets, as well as directly increasing the phagocytosis and bactericidal activity of abdominal macrophage in chicks (15).
Supplementation with (1→3)(1→6)-β-glucan has been shown to improve several base-line immune responses in the chicken (19). There is increased macrophage phagocytic activity with increases in nitrite and interleukin-1 production, enhanced antibody production, and an increase in the size of the primary and secondary lymphoid organs including the Bursa of Fabricius, thymus and spleen. This makes β-glucans potential immunomodulators for use in chicken production systems.
Dietary levels of (1→3)(1→6)-β-glucan above 0.02% were shown to improve growth performance, nutrient retention in addition to the improved immune function of broiler chickens (22). Yeast β-glucans were shown to be as effective as virginamycin in promoting growth of broiler chickens (24).
Shao et al. (25) looked at the effect of (1→3)(1→6)-β-glucan on the intestinal barrier structure and function. This intestinal barrier is a chief component of the innate immune system to keep intestinal pathogens from gaining entrance to the body. A Salmonella Typhimurium challenge can result in intestinal mucosal damage with a shortening, atrophy and reduced density of villi. Supplementation with (1→3)(1→6)-β-glucan increased villus height and villus height to crypt depth ratio in the jejunum after a Salmonella Typhimurium challenge. Villus height, crypt depth and the villus height to crypt depth ratio are important indicators of gut health. While the Salmonella Typimurium challenge damaged the integrity of the intestinal mucosa, the severity of the disruption was decreased after dietary supplementation of (1→3)(1→6)-β-glucan. There also was an increase in the number of goblet cells as well as secretory IgA.
When vaccinating animals, various substances can be used as the adjuvant. While 1% yeast β-glucan supplementation of diets fed to Pekin ducks did not improve duck growth, carcass composition or meat quality, the supplementation did enhance properties of the cellular component of the innate immune system (26).
Barley beta-glucans
As previously indicated, the molecular weights and degree of branching will affect the immune response of β-glucans. While (1→3)(1→6)-β-glucans from yeast and fungi have been successfully used to stimulate immune function in both mammals and birds, the effects of β-glucans from barley have not been as effective. Barley (1→3)(1→4)-β-glucans have been shown to induce only a mild immune response in vitro with mammalian macrophage cell lines (murine) with an increase in antibacterial lysozymes. Supplemented barley (1→3)(1→4)-β-glucans function as MAMPS and can be recognized by PPR dectin-1 which increases production of IL-6 and IL-8. (27-29). Barley β-glucans have also been shown to alter immune function of weanling pigs by increasing blood lymphocytes and the proportion of naïve T-cells. Unfortunately it also increased E. coli binding and intestinal permeability (30) so further research is needed before supplementing the diet of weanling pigs with barley β-glucans. Because of the negative effects of barley on performance, barley β-glucans have not been studied as an immunomodulator in poultry production. The adverse effects of barley β-glucans appear to be their propensity to increase intestinal viscosity. It would be of interest, however, to see if barley β-glucans have an effect on the immune system if the digestive tract is by-passed. For example, can barley β-glucans be used as an adjuvant for vaccines?
Conclusions
Beta-glucans are structural components of cell walls in many different sources including bacteria, fungi, algae and yeast. They are also found in grains such as oats and barley. The structure of the β-glucans, however, vary among the different sources and account for the differences in their physiological function. (1→3)(1→6)-β-glucans from yeast and fungi have highly branched structure and have been found to be the most effective types of β-glucans in terms of immune system stimulation.
Barley (1→3)(1→4)-β-glucans have the same (1→3)-β-linkages between the glycopyranosyl molecules that the β-glucans in yeast and fungi have, but the side branches are attached to the backbone with (1→4)-β-links. This difference in the linkages results in low water solubility and is the reason that barley β-glucans typically form gels in aqueous media.
Yeast β-glucans have been shown to enhance immune function in many different animals, including poultry. The β-glucans from barley, however, have a negative effect on bird performance and health.
With the increased pressure to reduce antibiotic use in food animal production, producers are looking at boosting immune function as an alternative approach to reducing the effects of intestinal pathogens. Beta-glucans from fungi and yeast may be ideal immunomodulators for poultry since they enhance the immune system without negatively affecting production performance. Barley β-glucans, however, have not shown this potential due to the adverse effects on poultry performance.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- White WB, Bird HR, Sunde ML, et al. The viscosity interaction of barley beta-glucan with Trichoderma viride cellulase in the chick intestine. Poult Sci 1981;60:1043-8. [PubMed]
- Jacob JP, Pescatore AJ. Using barley in poultry diets—a review. J Appl Poult Res 2012;21:915-40.
- Johansson L, Karesoja M, Ekholm P, et al. Comparison of the solution properties of (1→3),(1→4)-β-D-glucans extracted from oats and barley. LWT 2008;41:180-4.
- Kaiser P. Advances in avian immunology--prospects for disease control: a review. Avian Pathol 2010;39:309-24. [PubMed]
- Soruri A, Grigat J, Forssmann U, et al. Beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur J Immunol 2007;37:2474-86. [PubMed]
- Lynn DJ, Higgs R, Lloyd AT, et al. Avian beta-defensin nomenclature: a community proposed update. Immunol Lett 2007;110:86-9. [PubMed]
- Nicolas-Bolnet C, Qureshi MA, Cieszynski JA, et al. Avian hematopoiesis in response to avian cytokines. Poult Sci 1995;74:1970-6. [PubMed]
- Qureshi MA. Avian macrophage and immune response: an overview. Poult Sci 2003;82:691-8. [PubMed]
- Tizard I. Avian immune responses - a brief review. Avian Dis 1979;23:290-8.
- Du Z, Kelly E, Mecklenbräuker I, et al. Selective regulation of IL-10 signaling and function by zymosan. J Immunol 2006;176:4785-92. [PubMed]
- Rice PJ, Adams EL, Ozment-Skelton T, et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J Pharmacol Exp Ther 2005;314:1079-86. [PubMed]
- Suzuki I, Hashimoto K, Ohno N, et al. Immunomodulation by orally administered beta-glucan in mice. Int J Immunopharmacol 1989;11:761-9. [PubMed]
- Hetland G, Ohno N, Aaberge IS, et al. Protective effect of beta-glucan against systemic Streptococcus pneumoniae infection in mice. FEMS Immunol Med Microbiol 2000;27:111-6. [PubMed]
- Zhang B, Guo Y, Wang Z. The modulating effect of β-1,3/1,6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian-Aust J Anim Sci 2008;21:237-44.
- Chen KL, Weng BC, Chang MT, et al. Direct enhancement of the phagocytic and bactericidal capability of abdominal macrophage of chicks by beta-1,3-1,6-glucan. Poult Sci 2008;87:2242-9. [PubMed]
- Williams DL. Overview of (1→3)-beta-D-glucan immunobiology. Mediators Inflamm 1997;6:247-50. [PubMed]
- Tzianabos AO. Polysaccharide immunomodulators as therapeutic agents: structural aspects and biologic function. Clin Microbiol Rev 2000;13:523-33. [PubMed]
- Olson EJ, Standing JE, Griego-Harper N, et al. Fungal beta-glucan interacts with vitronectin and stimulates tumor necrosis factor alpha release from macrophages. Infect Immun 1996;64:3548-54. [PubMed]
- Guo Y, Ali RA, Qureshi MA. The influence of beta-glucan on immune responses in broiler chicks. Immunopharmacol Immunotoxicol 2003;25:461-72. [PubMed]
- Lowry VK, Farnell MB, Ferro PJ, et al. Purified beta-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int J Food Microbiol 2005;98:309-18. [PubMed]
- Chen HL, Li DF, Chang BY, et al. Effects of lentinan on broiler splenocyte proliferation, interleukin-2 production, and signal transduction. Poult Sci 2003;82:760-6. [PubMed]
- Chae BJ, Lohakare JD, Moon WK, et al. Effects of supplementation of beta-glucan on the growth performance and immunity in broilers. Res Vet Sci 2006;80:291-8. [PubMed]
- Huff GR, Huff WE, Rath NC, et al. Limited treatment with beta-1,3/1,6-glucan improves production values of broiler chickens challenged with Escherichia coli. Poult Sci 2006;85:613-8. [PubMed]
- Rathgeber BM, Budgell KL, MacIsaac JL, et al. Growth performance and spleen and bursa weight of broilers fed yeast beta-glucan. Can J Anim Sci 2008;88:469-73.
- Shao Y, Guo Y, Wang Z. β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult Sci 2013;92:1764-73. [PubMed]
- Tang XY, Gao JS, Yuan F, et al. Effects of Sophy β-glucan on growth performance, carcass traits, meat composition, and immunological responses of Peking ducks. Poult Sci 2011;90:737-45. [PubMed]
- Tada R, Adachi Y, Ishibashi K, et al. Binding capacity of a barley beta-D-glucan to the beta-glucan recognition molecule dectin-1. J Agric Food Chem 2008;56:1442-50. [PubMed]
- Tada R, Ikeda F, Aoki K, et al. Barley-derived beta-D-glucan induces immunostimulation via a dectin-1-mediated pathway. Immunol Lett 2009;123:144-8. [PubMed]
- Fan YG, Hu CW, Chu C, et al. Effect of barley β-glucan on murine RAW264.7 macrophages against virulent Salmonella enterica serovar Thyphimurium. Food Res Int 2012;45:1106-10.
- Ewaschuk JB, Johnson IR, Madsen KL, et al. Barley-derived β-glucans increases gut permeability, ex vivo epithelial cell binding to E. coli, and naive T-cell proportions in weanling pigs. J Anim Sci 2012;90:2652-62. [PubMed]