Immune-enhancing effects of Maitake (Grifola frondosa) and Shiitake (Lentinula edodes) extracts
Introduction
Natural products have been used to help treat and/or prevent diseases throughout man’s history. Natural [1,3]-β-D-glucans isolated from yeast, grain and mushrooms are well-established biological response modifiers [for review see (1)]. Alpha-glucan-containing substances also have demonstrated an ability to enhance immune functions (2). Glucans are highly conserved carbohydrates and represent a group of chemically heterogeneous polysaccharides existing in various numbers of molecules of glucose bound together with several types and degrees of branching.
Research on the relationship between glucan and immune reactions began approximately 70 years ago with two starting points: one originated in Europe and the United States and the second in Japan. Based mainly on historical use of materials used for isolation of glucan, the Japanese groups focused on mushroom-derived glucans and the European and American groups studied glucans isolated from Saccharomyces cerevisiae.
Initial glucan research led to the discovery of glucan’s ability to stimulate the phagocytic system, enhance general defense mechanisms and promote resistance to tumors. During subsequent decades of research around the world, glucans were found to significantly stimulate defense reactions against infections and cancer [for review see (3,4)]. In addition, several additional important effects were later demonstrated, including reduction of stress (5), hypoglycemic effects, reduction of cholesterol level (6), and improvements of ulcerative colitis (7) Another advantage of using glucan as both specific and nonspecific activator of immune reactions is the fact that it has been shown to be effective in all species tested so far—from earthworms to humans (8).
Both Maitake and Shiitake glucans are among the most studied glucans (9-15). The Maitake mushroom extract used in our study is rich in β-glucans, while Shiitake whole mushroom powder is α-glucan rich. Active hexose correlated compound (AHCC) is a α-glucan-rich nutraceutical ingredient prepared by culturing Shiitake and other Basidiomycetes mushrooms. AHCC was originally developed for lowering blood pressure, but significant immunostimulating effects were found later (16-18).
As some previously published studies have reported rather confusing results, we decided to evaluate the immunostimulating effects of Maitake mushroom extract, Shiitake whole mushroom powder, a blend of Maitake and Shiitake, as well as AHCC.
Materials and methods
Animals
Female, 8-week-old BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). All animal work was done according to the University of Louisville IACUC protocol. Animals were sacrificed by CO2 asphyxiation.
Materials
Trypan blue, RPMI 1640 medium, sodium citrate, LPS, Limulus lysate texs E-Toxate, polymixin B and Wright stain were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fetal calf serum (FCS) was from Hyclone Laboratories (Logan, UT, USA).
Glucans
MaitakeGold 404 (MTG404) extract is soluble glucan-rich extract isolated from Maitake fruit body (Grifola frondosa). The product, which is a glucan/protein complex, is derived by thermally extracting the fruit body of Maitake with water under pressure at 100 °C or more for 30 minutes to an hour. After that, alcohol is added to the extract at a final concentration of 20% to 60% by volume to remove floating material by filtration. The resulting extract is concentrated under heating to remove residual alcohol. The product is a hygroscopic powder in shades of brown which is soluble in water, alkaline solutions and dimethyl sulphoxide, with a molecular weight around 1,000 kD. MTG404 is produced by Yukiguni Maitake Co. (Niigata, Japan) and for this project was purchased from Tradeworks Group, Inc. (Brattleboro, Vermont, VT, USA).
The Shiitake mushroom used in this study is an alpha glucan-rich whole mushroom powder obtained from Gourmet Mushrooms, Inc. (Sebastopol, CA, USA). This mushroom is grown on brown rice, dried, and then ground into a fine powder.
AHCC (Amino-Up Chemical Company, Sapporo, Japan) is a proprietary, alpha glucan-rich nutraceutical ingredient prepared by culturing Shiitake and other Basidiomycetes mushrooms with rice bran and then subjecting the culture to an enzymatic reaction, followed by hot water extraction and freeze drying.
Oral treatment
Mice were treated orally twice/day for 14 days with either 61.5 μg/day MaitakeGold 404, 820 μg/day Shiitake, 820 μg Shiitake and 61.5 μg MaitakeGold 404, or 100 μg AHCC. PBS served as a negative control. The doses used in this study correspond to the recommended daily human-equivalent dose for each ingredient.
Phagocytosis
The technique employing phagocytosis of synthetic polymeric microspheres was described earlier (19,20). Briefly, peripheral blood cells were incubated with 0.05 mL of 2-hydroxyethyl methacrylate particles (HEMA; 5×108/mL). The test tubes were incubated at 37 °C for 60 min, with intermittent shaking. Smears were stained with Wright stain. The cells with three or more HEMA particles were considered positive. The same smears were also used for evaluation of cell types.
Evaluation of cytokine production
At the endpoints, blood was collected, serum prepared, filtered through 0.45 μm filters and tested for the presence of cytokines (IL-6, IL-12 and IFN-γ) using the Quantikine mouse IL-6, IL-12 or IFN-γ kit, respectively (R&D Systems, Minneapolis, MN, USA).
Evaluation of C-reactive protein (CRP) production
At the endpoints, blood was collected, serum prepared, filtered through 0.45 μm filters and tested for the presence of CRP using a CRP kit (Helica, Santa Anna, CA, USA).
NK cell assay
Spleen cells were isolated from the spleen of mice by standard methods. Cell suspension was generated by pressing minced spleen against the bottom of a petri dish containing PBS. After elimination of erythrocytes by 10-second incubation in distilled water, and five washes in cold PBS, the cells were resuspended in PBS and counted. The viability was determined by trypan blue exclusion. Only cells with viability better than 95% were used in subsequent experiments. Splenocytes (106/mL; 0.1 mL/well) in V-shaped 96-well microplates were washed three times with RPMI 1640 medium. After washing, 50 µL of target cell line YAC-1 (three different concentrations of target cells were used so the final effector-target ratio was 10:1, 50:1 and 100:1). After spinning the plates at 250 ×g for 5 min, the plates were incubated for 4 hr at 37 °C. The cytotoxic activity of cells was determined by the use of CytoTox 96 Non-Radioactive Cytotoxicity Assay from Promega (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Briefly, 10 µL of lysis solution was added into appropriate control wells 45 min before the end of incubation. The next step was to spin the plates at 250 ×g for 5 min, followed by transferring 50 µL of supernatant into flat-bottomed, 96-well microplates. After 50 µL of reconstituted substrate was added into each well, plates were covered and incubated for 30 min at room temperature at dark. The optical density was determined by using a STL ELISA reader (Tecan U.S., Research Triangle Park, NC) at 492 nm. Specific cell-mediated cytotoxicity was calculated using the formula:
Statistics
Student’s t-test was used to statistically analyze the data.
Results
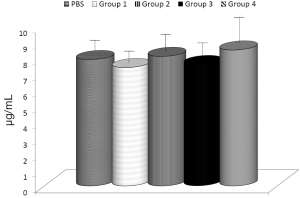
The effects of glucan on the phagocytic activity of professional phagocytes are well established. Therefore, a comparison of various glucans and/or their combinations should start with evaluation of how these compounds affect this reaction. We compared the activities of MaitakeGold 404, Shiitake, MTG404-Shiitake blend, and AHCC on phagocytosis of synthetic microspheres by peritoneal blood monocytes and neutrophils (Figure 1). With exception of AHCC and neutrophils, all tested samples significantly increased phagocytosis. The highest effects were observed in MaitakeGold 404-Shiitake combination.
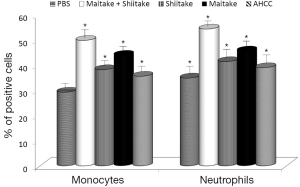
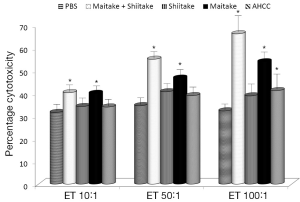
Next, we focused on activation of NK cells. Human YAC-1 cells were incubated with mouse spleen cells isolated from animals stimulated by tested samples (Figure 2). Our data showed that MaitakeGold 404-Shiitake combination and MaitakeGold 404 alone activated NK cell toxicity in all tested E:T ratios. AHCC was active only in the highest E:T ratio.
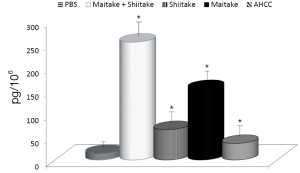
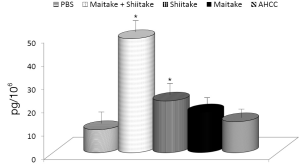
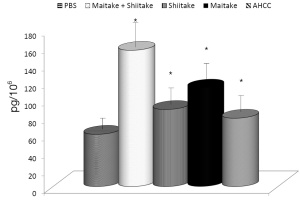
The effects on the cellular branch of immune reaction were followed by evaluation of possible activation of cytokine secretion. Serum obtained from mice fed with different samples was stored at –80 °C for no more than one week. The data summarized in Figure 3 showed that all samples significantly stimulated secretion of IL-6. The samples with the highest activity were MaitakeGold 404-Shiitake combination followed by MaitakeGold 404 alone. A different situation was found when we tested production of IL-12. Only MaitakeGold 404-Shiitake combination and Shiitake alone resulted in significantly elevated levels of IL-12 to spike to over twice the level of any other glucan and more than three times that of the PBS control (Figure 4). Effects on IFN-γ secretion were similar to those for IL-6 (Figure 5). CRP levels did not significantly change during the study (Figure 6).
Discussion
More than forty years ago, β-glucans were first described as biological response modifiers that could stimulate tumor rejection in mice. As with many other natural immunostimulators, they were classified as “non-specific” because their molecular targets were unknown and their effects appeared to be pleiotropic. Nevertheless, extensive literature regarding the activity of β-glucans in animal tumor models exists (4). For the past 30 years, in Japan, several forms of mushroom-derived β-glucan have been used successfully in cancer patients (21).
Despite decades of extensive research, the knowledge of the relation between physicochemical characteristics (such as molecular weight, degree of branching, conformation or solubility) and immunological activities is still limited. Even less is known about the relationship between α-glucans and immune reactions. With the continuing discussion whether yeast-derived glucans are better than mushroom-derived glucans (or vice-versa), and a rather limited number of research studies directly comparing individual glucans, the quest for the biologically optimal glucan continues.
Individual commercially available glucans significantly differ in their biological activities and subsequently in price (22). Additional information can be found, but the number of glucans directly compared is still seriously limited (23,24). This investigation focused on the biological and immunological properties of two individual mushroom-derived glucan-rich polysaccharides, MaitakeGold 404 (β-glucan rich) and Shiitake (α-glucan rich), a combination of these two ingredients, and α-glucan rich AHCC.
Glucans were originally developed as stimulators of cellular immunity; therefore, phagocytosis is always the method of choice for characterization of effects on immune reactions. For our study, we used the 2-hydroxyethyl methacrylate microspheres. These particles have only a very light negative charge resulting in no nonspecific adherence to the cell membrane (25).
Using both phagocytosis and NK cell activity as a model in our study, the combination of MaitakeGold 404 glucan and Shiitake glucan consistently showed stronger activities than either mushroom ingredient alone or AHCC. When we compared these glucans directly, MaitakeGold 404 glucan was always more active, despite a significantly lower dose.
In addition to the direct activation of cells involved in immune reactions, the immunostimulating effects of natural molecules such as glucans are directly and/or indirectly caused by potentiation of synthesis and subsequent secretion of various cytokines. Individual glucans significantly differ in their effects upon cytokine synthesis (26,27). We previously showed significant effects of MaitakeGold 404 glucan on cytokine formation (28).
In the present study, we found that the MaitakeGold 404-Shiitake combination significantly enhanced production of all three tested cytokines, IL-6, IL-12 and IFN-γ. In all cases, the MaitakeGold 404 by itself was more active than the Shiitake alone. As cytokine production is extremely sensitive to the presence of LPS, contamination with LPS might mask the real effects of glucan. Therefore, we used LPS-free samples (depleted of any potential LPS by addition of 10 μg/mL of polymixin B) in parallel to normal samples. In each case, no significant difference in activity between normal and LPS-free sample was found (data not shown).
Our findings of levels of CRP showed no significant changes. These results correspond to the recently published study testing glucan effects on CRP levels in saliva of children with chronic respiratory problems (Richter 1).
Our current study clearly demonstrates that Maitake-derived glucan act via the same mechanisms as other highly active glucans to strongly stimulate immune defense reactions and, when combined with Shiitake glucan, the MaitakeGold 404-Shiitake blend is even more immunologically and biologically active than either glucan alone or AHCC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vetvicka V, Novak M. eds. Biology and Chemistry of Beta Glucan, Vol. 2, Beta-glucan, Structure, Chemistry and Specific Application. Oak Park: Bentham Science Publ., 2013.
- Roman BE, Beli E, Duriancik DM, et al. Short-term supplementation with active hexose correlated compound improves the antibody response to influenza B vaccine. Nutr Res 2013;33:12-7. [PubMed]
- Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 2008;5:47-57. [PubMed]
- Novak M, Vetvicka V. Glucans as biological response modifiers. Endocr Metab Immune Disord Drug Targets 2009;9:67-75. [PubMed]
- Vetvicka V, Vetvickova J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N Am J Med Sci 2011;3:320-4. [PubMed]
- Rahar S, Swami G, Nagpal N, et al. Preparation, characterization, and biological properties of β-glucans. J Adv Pharm Technol Res 2011;2:94-103. [PubMed]
- Lavi I, Levinson D, Peri I, et al. Orally administered glucans from the edible mushroom Pleurotus pulmonarius reduce acute inflammation in dextran sulfate sodium-induced experimental colitis. Br J Nutr 2010;103:393-402. [PubMed]
- Vetvicka V, Sima P. β-Glucan in invertebrates. Inv Surv J 2004;1:60-5.
- Mizuno T, Zhuang C. Maitake, Grifola frondosa, pharmacological effects. Food Rev Int 1995;11:135-49.
- Kodama N, Yamada M, Nanba H. Addition of Maitake D-fraction reduces the effective dosage of vancomycin for the treatment of Listeria-infected mice. Jpn J Pharmacol 2001;87:327-32. [PubMed]
- Lin H, She YH, Cassileth BR, et al. Maitake beta-glucan MD-fraction enhances bone marrow colony formation and reduces doxorubicin toxicity in vitro. Int Immunopharmacol 2004;4:91-9. [PubMed]
- Deng G, Lin H, Seidman A, et al. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J Cancer Res Clin Oncol 2009;135:1215-21. [PubMed]
- Rincão VP, Yamamoto KA, Ricardo NM, et al. Polysaccharide and extracts from Lentinula edodes: structural features and antiviral activity. Virol J 2012;9:37. [PubMed]
- Ina K, Kataoka T, Ando T. The use of lentinan for treating gastric cancer. Anticancer Agents Med Chem 2013;13:681-8. [PubMed]
- Finimundy TC, Gambato G, Fontana R, et al. Aqueous extracts of Lentinula edodes and Pleurotus sajor-caju exhibit high antioxidant capability and promising in vitro antitumor activity. Nutr Res 2013;33:76-84. [PubMed]
- Ulbricht C, Brigham A, Bryan JK, et al. An evidence-based systematic review of active hexose correlated compound (AHCC) by the Natural Standard Research Collaboration. J Diet Suppl 2013;10:264-308. [PubMed]
- Daddaoua A, Martínez-Plata E, Ortega-González M, et al. The nutritional supplement Active Hexose Correlated Compound (AHCC) has direct immunomodulatory actions on intestinal epithelial cells and macrophages involving TLR/MyD88 and NF-κB/MAPK activation. Food Chem 2013;136:1288-95. [PubMed]
- Shigama K, Nakaya A, Wakame K, et al. Alleviating effect of active hexose correlated compound (AHCC) for anticancer drug-induced side effects in non-tumor-bearing mice. J Exp Ther Oncol 2009;8:43-51. [PubMed]
- Vĕtvicka V, Fornůsek L, Kopecek J, et al. Phagocytosis of human blood leukocytes: a simple micromethod. Immunol Lett 1982;5:97-100. [PubMed]
- Vĕtvicka V, Holub M, Kovárů H, et al. Alpha-fetoprotein and phagocytosis in athymic nude mice. Immunol Lett 1988;19:95-8. [PubMed]
- Hazama S, Watanabe S, Ohashi M, et al. Efficacy of orally administered superfine dispersed lentinan (beta-1,3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res 2009;29:2611-7. [PubMed]
- Vetvicka V, Vetvickova J. β-1,3-Glucan: silver bullet or hot air? Open Glycoscience 2010;3:1-6.
- Vetvicka V, Vetvickova J. An evaluation of the immunological activities of commercially available β-1,3-glucans. J Am Nutr Assoc 2007;10:25-31.
- Vetvicka V, Vetvickova J. Physiological effects of different types of beta-glucan. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2007;151:225-31. [PubMed]
- Vĕtvicka V, Fornůsek L. Polymer microbeads in immunology. Biomaterials 1987;8:341-5. [PubMed]
- Adachi Y, Okazaki M, Ohno N, et al. Enhancement of cytokine production by macrophages stimulated with (1-->3)-beta-D-glucan, grifolan (GRN), isolated from Grifola frondosa. Biol Pharm Bull 1994;17:1554-60. [PubMed]
- Hirata N, Tsuzuki A, Ohno N, et al. Cytokine synthesis of human monocytes stimulated by triple or single helical conformer of an antitumour (1-->3)-beta-D-glucan preparation, sonifilan. Zentralbl Bakteriol 1998;288:403-13. [PubMed]
- Vetvicka V, Vetvickova J. Immunostimulating properties of two different)-β-glucans isolated from Maitake mushrooms (Grifola frondosa). JANA 2005;8:33-9.