
A novel intrauterine stent for prevention of intrauterine adhesions
Introduction
Intrauterine adhesions (IUAs), also known as Asherman syndrome, was reported and studied for more than a hundred years (1). The prevalence ranges from 1.73% to 40%, and the symptoms include hypomenorrhea, amenorrhea, and periodic lower quadrant abdominal pain. IUAs affect pregnancy, having various presentations; for example, infertility, repeated pregnancy loss, poor implantation, and abnormal placentation (2-6). Surgery has been the standard treatment choice for the management of Asherman syndrome. Since surgical wounds are prone to re-adhesion, the postoperative recurrence rate is as high as 3.1–62.5% (2,7,8). In practice, surgeons have tried to use a series of solid and semi-solid barriers as secondary prevention strategies to supply an artificial interlayer between the uterine walls to prevent their fusion during the initial healing phase.
Adding on, the uses of IUD with different shapes were also described as a possible treatment option. However, copper-containing uterine-shape and T-shape IUD can induce inflammation and are thus not recommended because of their insufficient surface area. Since there are many devices that do not fit well with the shape of the uterine cavity, the uterine cavity may be deformed after insertion of devices like an O-shape IUD and a Lippes loop (9-11). Also, there are no sizes of IUD available for too large or too small uterine cavities. IUD may be displaced surrounded or even detached, especially if the IUD is larger or smaller than the uterine cavity.
For the Foley catheter with a balloon at the top, if the balloon is too large, it can easily compress the endometrium and cause ischemia necrosis. On the other hand, if it is too small, it can fall off within minutes after inserting it. On the flip side, it can also prevent uterine and/or cervical canal adhesion, which are the main concerns of catheter indwelling. Such as uterine perforation, the ascending infection from the vagina and the high discomfort (12). Semi-solid barriers, usually referred to as hyaluronic acid gel, may not be suitable alone for endometrial surfaces due to a short half-life and weak attachment to the endometrium (13). The material usually needs to be used in combination with other devices. Comprehensive auxiliary therapy has become a consensus of subsequent adhesion reformation after adhesiolysis due to their defects, like examples mentioned above. So far, there is no effective and ideal anti-adhesion barrier. Here, we described our development of a uterine-shape device that does not damage the endometrium and has enough effective area to completely separate the anterior intrauterine wall from the posterior wall and maintain the shape of the uterine cavity. Our proposed design may result in a low-cost, long-term, and wide-spread solution for the use in patients with IUA.
The hypothesis
The manufacture of the uterine stent (see Figure 1)
- The uterine-shaped stent takes medical silicone rubber as raw materials. Silicone rubber materials have been widely used for biomedical applications due to its stable chemical properties, excellent heat resistance, ozone aging resistance, flux resistance, and high permeability. Earlier studies have verified that silicone rubber has good histocompatibility and no cytotoxicity (14,15). The lightweight and elastic materials can be rolled up to make them easy to place and remove through the narrow cervical canal and remain firmly in the uterine cavity.
- The stent has a thin film less than 0.4 mm in thickness in the center and periphery stiffener about 2 mm thick to supply enough area to separate the uterine walls without changing the shape of the stent. The thin central film will not compress the endometrium or result in serious endometrial necrosis. The stiffener ensures enough strength to support the device without squeezing the central film and deforming in the uterine cavity. Then the dilated space is conducive to endometrium regeneration, or repairment is of vital importance.
- Holes at both ends of the membrane are of 2–4 mm in diameter, which is convenient for installation and insertion into the uterus under the push of the pushrod. The flow of secretions on both sides is not restricted.
- The lower part of the stent has three grooves to facilitate the drainage of menstrual blood or fluid, which helps relieve abdominal pain and reduce the incidence of infection.
- Besides, there is a lower tail with a triangular shape to prevent and treat lower uterine segment and cervical canal adhesion. The tail may prevent adhesions of the lower part of the uterine cavity and the cervix canal. The end is a cylindrical tail with a diameter of 1 mm and a length of up to 30 mm. The length of the columnar tail can be determined based on whether the patient has cervical canal adhesion or not.
- There are more sizes to choose from. Varied sizes of stent were selected according to the distance between the two uterine angles.
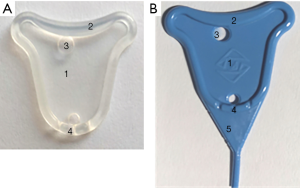
In this design, the uterine stent fits into the normal shape of the uterine cavity and keeps the normal uterine cavity shape after surgery. It supplies enough effective physical barriers, even to the cervical canal. Fluent uterine outflow channels can ease the discharge of uterine cavity effusion or blood, which helps to restore menstruation, reduce abdominal symptoms and inflammatory reaction. The stent does not compress or damage the endometrium, widening the uterine cavity volume is beneficial for the endometrium to regenerate and repair. A follow-up hysteroscopy is carried out 2–4 months after HA, and the uterine stent may be removed using a 5 Fr or 7 Fr rigid double-action forceps hysteroscopically.
Testing the hypothesis
At present, we have conducted some experiments on black goats. Goat is a bicornuate uterus animal, and hysteroscopy and surgery can be performed on goats. The experiments were approved by the Third Xiangya Hospital Laboratory Animal Management and Ethics Committee, and followed the Principles of Care and Use of Laboratory Animals of China. At first, uterine adhesions were caused by hysteroscopic electrocoagulation of goat endometrium. Three months later, slight adhesions were found at the lateral wall by hysteroscopy, and then the novel stent was successfully placed into the uterine cavity of a goat after HA treatment. During the placement, the life habit of the goat was the same as before, and no obvious vaginal bleeding was observed. The bacterial culture results of the cervical secretions were negative after two days of culture. Hysteroscopy was performed three months later. The results manifested that there was no obvious adhesion formation in the uterine cavity and the uterine stent was in the same position as before. One month after the stent was removed, the goat urine HCG was tested positive, and the pregnant sac was seen in the uterus through hysteroscopy (see Figure 2). Unfortunately, it has not been confirmed in large sample animal studies.
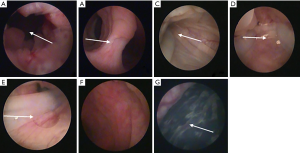
Animal experiments verified the safety and effectiveness of the new uterine stent. However, it is extremely important to evaluate the safety and efficacy of the new instrument of IUAs by a prospective, multicenter, randomized, parallel-controlled study. We are going to start a clinical trial, and the design is as follows: patients with IUAs were randomly divided into experimental group and control group, in the experimental group the uterine stent was used following adhesiolysis, and in the control group the traditional IUD group and the hyaluronic acid gel was inserted. Reassessment of the uterine cavity was carried out for both groups, usually via hysteroscopy after three menstrual cycles. The effectiveness and safety of implication in the management of IUAs will be evaluated by comparing the AFS score, recurrence rate, menstrual recovery rate, pregnancy rate and pregnancy outcomes between two groups. Ultrasonography will be used to monitor the endometrial thickness and intrauterine effusion.
Application of the device
Due to the refractory nature of uterine adhesions, it is becoming one of the controversial and challenging issues in the field of gynecology and reproduction. All patients undergoing surgery must be counseled about the possibility of repeat surgery. In general, patients with severe IUAs often require several repeated procedures because of the difficult nature of the procedure and the high rate of recurrent adhesions (2). In theory, this new type of uterine stent, as one of the best alternatives, is safe and effective in the management of IUAs. Its unique advantage is that it separates the anterior uterine cavity wall from the posterior wall, promotes the drainage of uterine fluid, thus effectively reducing or preventing the recurrence of the uterine cavity and/or cervix canal adhesions. Different models and size options make uterine stents suitable for more patients. We expect that the new uterine stents should effectively reduce the surgical operation frequency and times and lessen the financial burden and mental stress of patients. We will further test and improve the current device and develop a new generation of products. The novel uterine stent will be widely used in clinics after the theoretical and clinical practice verification, bringing new hopes for numerous patients with Asherman syndrome.
Acknowledgments
Funding: This study is supported by the Natural Science Foundation of China (Grant No. 81671492) and the Fundamental Research Funds for the Central Universities of Central South University (No. 2018zzts265).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Asherman JG. Traumatic intrauterine adhesions. J Obstet and Gynaecol Br Emp 1950;57:892-6. [Crossref] [PubMed]
- Yu D, Wong YM, Cheong Y. Asherman syndrome--one century later. Fertil Steril 2008;89:759-79. [Crossref] [PubMed]
- Westendorp IC, Ankum WM, Mol BW, et al. Prevalence of Asherman’s syndrome after secondary removal of placental remnants or a repeat curettage for incomplete abortion. Hum Reprod 1998;13:3347-50. [Crossref] [PubMed]
- Schenker JG, Margalioth EJ. Intrauterine adhesions: an updated appraisal. Fertil Steril 1982;37:593-610. [Crossref] [PubMed]
- Yucebilgin MS, Aktan E, Bozkurt K, et al. Comparison of hydrosonography and diagnostic hysteroscopy in the evaluation of infertile patients. Clin Exp Obstet Gynecol 2004;31:56-8. [PubMed]
- Hooker AB, Lemmers M, Thurkow AL, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update 2014;20:262-78. [Crossref] [PubMed]
- Yu D, Li TC, Xia E, et al. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman’s syndrome. Fertil Steril 2008;89:715-22. [Crossref] [PubMed]
- Roy KK, Baruah J, Sharma JB, et al. Reproductive outcome following hysteroscopic adhesiolysis in patients with infertility due to Asherman’s syndrome. Arch Gynecol Obstet 2010;281:355-61. [Crossref] [PubMed]
- Vesce F, Jorizzo G, Bianciotto A, et al. Use of the copper intrauterine device in the management of secondary amenorrhea. Fertil Steril 2000;73:162-5. [Crossref] [PubMed]
- March CM, Israel R. Gestational outcome following hysteroscopic lysis of adhesions. Fertil Steril 1981;36:455-9. [Crossref] [PubMed]
- Salma U, Xue M, Md Sayed AS, et al. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomed Res Int 2014;2014:589296. [Crossref] [PubMed]
- Conforti A, Alviggi C, Mollo A, et al. The management of Asherman syndrome: A review of literature. Reprod Biol Endocrinol 2013;11:118. [Crossref] [PubMed]
- Guida M, Acunzo G, Di Spiezio Sardo A, et al. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: a prospective, randomized, controlled study. Hum Reprod 2004;19:1461-4. [Crossref] [PubMed]
- Chen Y, Luo Y, Jia Z, et al. Preparation and characterization of silicone rubber/nano-copper nanocomposites for use in intrauterine devices. Biomed Mater Eng 2014;24:1269-74. [Crossref] [PubMed]
- Vince V, Thil MA, Veraart C, et al. Biocompatibility of platinum-metallized silicone rubber: in vivo and in vitro evaluation. J Biomater Sci Polym Ed 2004;15:173-88. [Crossref] [PubMed]


