
Long-term outcome of unilateral deep brain stimulation of the subthalamic nucleus for a patient with drug-resistant focal myoclonic seizure
Introduction
Epilepsy is one of the most common chronic neurological disorders, and approximately one-third of patients with focal epilepsy have persistent seizures despite adequate treatment with anti-epileptic drugs. Surgical resection is recognized as the powerful treatment method for drug-resistant focal epilepsy with a precisely delineated epileptogenic zone. However, surgical resection is not feasible for patients in whom the epileptic foci reside in the eloquent cortex of the brain (1,2). In particular, surgical resection can be at high risk of causing irreversible neurological impairments for patients with motor seizures arising from the primary motor cortex (3,4). Over the past few decades, the therapeutic role of deep brain stimulation (DBS) has been reported in patients with refractory seizures. While the anterior nucleus of the thalamus has been proven to be a promising target for focal drug-resistant epilepsy, especially temporal lobe epilepsy, by a multicenter, double‐blind, randomized controlled trial (5,6), the specific target for motor seizures needs to be investigated (7,8).
There is a consensus that the subthalamic nucleus (STN) plays a critical role in the cortical-basal ganglia motor loop (9,10). Clinically, STN-DBS has been successfully used to improve motor symptoms such as tremors, rigidity, and bradykinesia in patients with movement disorders (11). In recent years, some animal experiments have shown that the STN is also involved in the process of motor seizures, suggesting that the STN might be a promising site for DBS for patients with focal motor seizures (12,13). Here, we report a 5-year follow-up of a patient with drug-resistant focal myoclonic seizures treated by unilateral STN-DBS and the case report is presented in accordance with the CARE Guideline.
Case presentation
The patient was a right-handed 13-year-old boy without any personal or familial antecedents for epilepsy. Ictal episodes of brief, frequent myoclonic jerks of the right fingers started at 6 years old, which could progress into rhythmic clonic jerks over the right arm or hemibody. In recent years, he suffered from intermitted epilepsia partialis continua despite optional poly-antiepileptic drug therapy. Obvious weakness of the right arm and slight weakness of the right lower limb were detected. MRI showed subtle atrophy in the left frontal and central regions. Interictal EEG showed suspected low amplitude spiking in the left frontocentral area, but no clear findings on ictal EEG due to massive muscle artifacts were observed. Magnetoencephalography (MEG) revealed dipole clusters over the left central area (Figure 1). In summary, a seizure onset zone involving the left primary motor cortex was suspected. Unilateral STN-DBS for this patient was designed to treat epilepsia partialis continua.
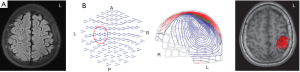
One DBS lead (model 3389; Medtronic) with four platinum-iridium cylindrical surface contacts of 1.27-mm diameter and 1.5-mm length were spaced 0.5 mm apart and implanted into the left STN in 2013. Contacts 0 and 3 were the lowermost and uppermost contacts, respectively. The DBS lead targeted the dorsolateral area of the STN. Based on the classical anatomical location of the STN, stereotaxic coordinates of the STN were refined by direct visualization in the individual T2-weighted MRI. LEAD-DBS software (www.lead-dbs.org) was used to reconstruct the DBS lead (14). In brief, the postoperative MRI was linearly coregistered with the preoperative MRI. The results of the coregistrations were manually adjusted and refined for the patient. Then, the coregistered MRI scans were nonlinearly normalized into the standardized MNI stereotactic space to reconstruct contact locations. The location of each electrode’s contact was thereby registered in MNI space (Figure 2). The subdivisions including the motor, the associative and the limbic area of the STN were displayed using DISTAL atlas (DBS Intrinsic Template Atlas) (15).

The STN was continuously stimulated after 3 months of permanent implantation of the DBS lead. Further adjustments of the stimulation parameters were scheduled if clinical worsening was reported at every 12-month follow-up visit. Seizure frequency, epilepsy severity, and life satisfaction were evaluated at each visit and compared with those of the baseline period (three months preceding surgery). At the final follow-up 5 years after the procedure, the patient experienced efficacious improvements in seizure frequency, seizure severity, and life satisfaction (Figure 3). DBS began with bipolar, unilateral stimulation with a current of 0.5 mA, a frequency of 130 Hz, and a 90 µs pulse width (at contact 0−, 2+). The patient experienced an accidental DBS interruption at 21 months after initial stimulation. To obtain a better outcome, two monopolar configurations with frequencies of 33 and 160 Hz (2-C+, 90 µs) were applied at 24 and 36 months, respectively. After the initiation of STN-DBS, the mean percentage reductions in disabling seizure frequency compared to baseline were 50%, 40%, 70%, 90%, and 75% at each corresponding 12-month follow-up over the 5-year period. Accordingly, the epilepsy severity scores (Worst, 10) were 4, 5, 4, 1 and 2, respectively, and the life satisfaction scores (Best, 10) were 7, 6, 8, 9 and 8 on a scale from 1 to 10.

This study was approved by the Institutional Review Board Committee in accordance with the ethical standards of the Declaration of Helsinki, and informed consent was obtained from the patient.
Discussion
Because available anti-epileptic drugs have failed to control seizures and remarkable impairment of motor function is foreseeable if resection surgery is performed, the patient in this study underwent STN-DBS to treat motor seizures. The five-year long-term clinical outcome demonstrated the stable therapeutically modulatory effects of high frequency stimulation (HFS) of the STN. In line with the decreased seizure frequency and severity, quality of life also improved dramatically. To date, only a few patients with motor seizures who have been treated by STN-DBS have been reported. In 2001, Benabid and his colleagues first reported that HFS of the STN was performed in five patients suffering from medically intractable seizures, and a 67% to 80% reduction in seizure frequency was observed in three of the patients with focal seizures originating from the central area (16). Another study showed that bilateral monopolar STN-DBS reduced the intensity and frequency of seizures by 50% in a patient with progressive myoclonic epilepsy (17). Thus, together with the previous research, our data provide further evidence that STN-DBS has a good effect on cases of focal motor seizures.
The potential role of STN-DBS in motor seizures has also been suggested by experimental studies. Knowledge of the pathophysiology of Parkinsonism is burgeoning, which has contributed greatly to the understanding of the cortical-basal ganglia loop in motor processing (18). Some experimental data has shown the involvement of the motor part of basal ganglia nuclei in focal motor seizures. Recently, Devergnas et al. investigated the subcortical circuits of motor seizures (13). They studied the activity of basal ganglia during focal motor seizures experimentally induced by the injection of penicillin within the primary motor cortex, and they proposed a global portrait of the propagation pathways within the basal ganglia for focal motor seizures, demonstrating that the subthalamo-(external)-pallidal pathway was the main subcortical route involved during ictal motor seizures. Another animal study indicated that HFS-STN improved focal motor seizures by delaying the occurrence of the first seizure and decreasing the number of seizures compared with the control group (12).
The mechanism of the action of STN-DBS for motor seizures is unclear. In a prior study (19), we showed that high-frequency stimulation of the anterior nucleus of the thalamus could desynchronize focal and large-scale epileptic networks and thereby reduce seizure generation and propagation. We thereby speculate that HFS-STN could modify pathological cortical hypersynchronization of the motor cortex and therefore could reduce seizure activity.
Overall, our report provides new evidence that the STN is a potential target for the treatment of focal motor epilepsy, which might offer an option for patients with drug-resistant motor seizures.
Acknowledgments
The authors would like to thank Wei Du, Xiaoxia Zhou, Yuanyuan Piao, Yanran Li, and Di Wu for the technical assistant.
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 81571271, 81771395, 81771398).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board Committee in accordance with the ethical standards of the Declaration of Helsinki, and informed consent was obtained from the patient.
References
- Jobst BC, Cascino GD. Resective epilepsy surgery for drug-resistant focal epilepsy: a review. JAMA 2015;313:285-93. [Crossref] [PubMed]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain 2001;124:1683-700. [Crossref] [PubMed]
- Benifla M, Sala F Jr, Jane J, et al. Neurosurgical management of intractable rolandic epilepsy in children: role of resection in eloquent cortex. Clinical article. J Neurosurg Pediatr 2009;4:199-216. [Crossref] [PubMed]
- Rolston JD, Englot DJ, Knowlton RC, et al. Rate and complications of adult epilepsy surgery in North America: Analysis of multiple databases. Epilepsy Res 2016;124:55-62. [Crossref] [PubMed]
- Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 2010;51:899-908. [Crossref] [PubMed]
- Ren L, Kucewicz MT, Cimbalnik J, et al. Gamma oscillations precede interictal epileptiform spikes in the seizure onset zone. Neurology 2015;84:602-8. [Crossref] [PubMed]
- Fisher RS, Velasco AL. Electrical brain stimulation for epilepsy. Nat Rev Neurol 2014;10:261-70. [Crossref] [PubMed]
- Sprengers M, Vonck K, Carrette E, et al. Deep brain and cortical stimulation for epilepsy. Cochrane Database Syst Rev 2017;7:CD008497. [PubMed]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989;12:366-75. [Crossref] [PubMed]
- Okun MS. Deep-brain stimulation--entering the era of human neural-network modulation. N Engl J Med 2014;371:1369-73. [Crossref] [PubMed]
- Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med 2012;367:1529-38. [Crossref] [PubMed]
- Prabhu S, Chabardès S, Sherdil A, et al. Effect of subthalamic nucleus stimulation on penicillin induced focal motor seizures in primate. Brain Stimul 2015;8:177-84. [Crossref] [PubMed]
- Devergnas A, Piallat B, Prabhu S, et al. The subcortical hidden side of focal motor seizures: evidence from micro-recordings and local field potentials. Brain 2012;135:2263-76. [Crossref] [PubMed]
- Horn A, Li N, Dembek TA, et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019;184:293-316. [Crossref] [PubMed]
- Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 2018;170:271-82. [Crossref] [PubMed]
- Benabid AL, Koudsie A, Benazzouz A, et al. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson's disease. Extension to new indications such as dystonia and epilepsy. J Neurol 2001;248 Suppl 3:III37-47. [Crossref] [PubMed]
- Wille C, Steinhoff BJ, Altenmüller DM, et al. Chronic high-frequency deep-brain stimulation in progressive myoclonic epilepsy in adulthood--report of five cases. Epilepsia 2011;52:489-96. [Crossref] [PubMed]
- Dayal V, Limousin P, Foltynie T. Subthalamic Nucleus Deep Brain Stimulation in Parkinson's Disease: The Effect of Varying Stimulation Parameters. J Parkinsons Dis 2017;7:235-45. [Crossref] [PubMed]
- Yu T, Wang X, Li Y, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain 2018;141:2631-43. [PubMed]


