
Liver impairment in critical illness and sepsis: the dawn of new biomarkers?
The acute onset of liver impairment is frequently seen in critical illness and sepsis. However, the exact mechanisms for the hepatic deterioration are not completely understood. Also, the impact of liver dysfunction in critically ill patients on the clinical outcome has been debated over the past years. Due to the versatile functions the liver has to fulfil, it remains challenging to accurately assess liver impairment, since virtually no single parameter completely displays the multiplicity of liver functions. Bilirubin is the standard parameter for the assessment of liver dysfunction in critical care medicine. Elevated bilirubin reflects impairment in the metabolic process of bile formation, bile secretion and reduced bile flow in the biliary tract (1). However, the weakness of bilirubin as a parameter for liver impairment might be the considerable time lag between hepatic injury and development of hyperbilirubinemia (2). A recent study by Jensen and coworkers revisited the role of acute liver impairment in critical illness by analysing several biomarkers in a large, multi-center patient cohort (n=1,096) and by linking the findings to mortality (3). While the authors confirmed that bilirubin is an independent predictor of 90-day mortality, they identified serum levels of hyaluronic acid (HA) as a particular risk factor for mortality in patients with infections (3).
HA as a biomarker for fibrosis, cirrhosis and impaired liver function
HA is a glycosaminoglycan of high molecular weight and a component of the extracellular matrix of most tissues in the human body. In the liver, HA is synthesized by stellate cells, the matrix producing fibroblast population. In addition, connective tissue outside the liver releases HA, which is transported via lymphatic vessels to the bloodstream and almost exclusively degraded by sinusoidal endothelial cells of the liver (4,5). Studies tracing radioactive HA showed that HA has a plasma half-life time of approximately 2.5 to 5.5 min in healthy individuals (6). Due to the fact that the liver is the main site of HA removal from circulation and because of its short half-life time, HA was regarded as a blood parameter for liver impairment and parenchymal liver damage early on, especially for liver fibrosis. Indeed, the first studies evaluating HA as a marker for liver fibrosis and cirrhosis were conducted more than three decades ago (6,7). Over the past 30 years, HA has been extensively studied as a non-invasive biomarker for fibrosis in various liver diseases such as alcoholic liver disease, non-alcoholic fatty liver disease, viral hepatitis, primary biliary cirrhosis as well as autoimmune, drug-induced or chemical induced liver injury (5). In those studies, HA appears to correlate well with the degree of fibrosis in patients with chronic liver disease, likely reflecting the increased deposition of extracellular matrix in the liver and the reduced clearance of HA by the sinusoidal endothelial cells of the liver. To date HA is used in several scoring systems, such as the Enhanced Liver Fibrosis (ELF) score, in order to assess liver fibrosis in a non-invasive manner (5). More recently, there have been several studies evaluating HA as a prognostic parameter in patients with chronic liver disease in order to predict mortality (8), the risk of allograft failure in liver transplanted patients (9) or to predict the outcome in acute liver failure with no underlying chronic liver disease (10). Jensen and colleagues now tested the hypothesis that HA could indicate already mild liver impairment in critically ill patients (3). Of note, HA is known to be elevated in conditions of sepsis and septic shock, most likely due to reduced clearance by the sinusoidal endothelial cells, but increased HA levels might also be related to specific mechanisms in sepsis such as HA release from bacterial walls (11). Eventually, the exact regulation of HA levels in critical illness remains elusive and the question if HA directly reflects liver impairment under these conditions is not definitely clarified, yet.
The role of liver impairment in patients with critical illness
The role of pre-existing liver dysfunction, such as liver cirrhosis, in patients admitted to intensive care units (ICUs) is widely acknowledged, as it is associated with a poor outcome and high mortality (12,13). For instance, in a monocentric retrospective study, patients with cirrhosis admitted to the ICU had a 1-year mortality rate of 89%, if they required mechanical ventilation (14). However, the adverse prognosis of cirrhotic patients at the ICU is largely related to the concomitant development of multiple organ failure(s), including the liver, termed acute-on-chronic liver failure (ACLF). If critically ill ACLF patients are properly matched to non-ACLF patients with organ failure(s), their prognosis is similarly poor (15).
The meaning of mild or moderate hepatic dysfunction in absence of an underlying liver disease for the risk of mortality, however, is less clear. Hepatic dysfunction is routinely assessed on ICUs as part of the sequential organ failure assessment (SOFA) score, in which serum bilirubin is included. Impairment of hepatic function is frequently seen in patients either at the point of ICU admission or in the course of critical care treatment. Simplified, two pathophysiological conditions are usually distinguished in terms of clinical appearance and laboratory assessment. Firstly, ischemic hepatitis displays itself as diffuse hepatocellular necrosis provoked by acute hypoxemia as a result of reduced (arterial) blood supply in critical illness. Ischemic hepatitis is supposed to occur in 5-10% of critically ill patients (16,17). Secondly, cholestatic liver dysfunction, which is defined by impaired bile formation and excretion, is in critically ill patients usually not initially caused by obstruction of bile ducts but by non-obstructive accumulation of bile acids and bilirubin in the liver. This “critical illness” cholestatic liver dysfunction is usually defined by serum bilirubin level >2 mg/dL, mostly accompanied by increased levels of alkaline phosphatase (ALP). This type of liver function is supposed to occur in approximately 20% of all patients on intensive care units (17). Previous studies reported that hepatic dysfunction occurs in approximately 11–31% of critically ill patients (18,19) and therefore has to be assumed to be a frequent complication in critical care medicine. Since hepatic dysfunction is less immediately life-threatening compared to renal, respiratory or cardiac failure, it seems that less attention has been paid to this complication. Also, the actual impact of liver impairment in the setting of intensive care medicine on mortality and morbidity has been controversially debated, ranging from the assumption that liver impairment has no direct effect on mortality (18) to the statement that it harbours a greater risk for mortality than every other single organ failure, namely renal, cardiovascular, respiratory and haematological dysfunction (19). Considering this, it is rather astonishing how little is known about the influence of early hepatic dysfunction in intensive care medicine; this highlights the importance of studies like the one recently presented by Jensen and colleagues (3).
In this study, the investigators assessed hepatic dysfunction by measuring circulating biomarkers such as HA, bilirubin, ALP and international normalized ratio (INR) in 1,096 medical and surgical ICU patients without pre-existing chronic liver disease that had been enrolled into a prospective PCT-related biomarker study (20). They found a significant correlation between HA as well as bilirubin with all-cause mortality in critically ill patients (Table 1). Furthermore, they could show in sub-group analyses that critically ill patients without infections [defined as procalcitonin (PCT) <1.0 ng/mL] showed significantly lower HA levels than those with an infection (PCT >1.0 ng/mL). Also, the correlation between HA and mortality did not count for the sub-group of non-infected patients, while in the group of critically ill infected patients HA showed a quartile-wise direct correlation to the risk of dying within the first 90 days after admission to the intensive care unit. This relationship between mortality in the group of infected patients could also be shown for bilirubin, whereas, opposite to HA, the increase of risk between the 1st and 2nd quartile did not reach statistical significance in univariate analysis. An earlier study by Kramer and coworkers addressing the role of hepatic impairment used solely bilirubin as biomarker for liver impairment (Table 1) (19). This study had a larger study cohort and included a total of 4,146 patients with early hepatic dysfunction (out of 38,036 consecutive patients) upon ICU admission and came to comparable results. Liver impairment indicated by increased bilirubin levels was a strong and independent indicator for higher hospital mortality (19). Interestingly, in this study sepsis was significantly more frequent in the group of patients with hepatic dysfunction than in the control group. Jensen et al. hypothesized that liver dysfunction leads to dysregulation in the inflammatory response, resulting in increased susceptibility for (bacterial) infections and therefore higher mortality risk especially in critically ill patients with severe infections (3). This conclusion might be hasty, since sepsis could just as well cause hepatic impairment and therefore contribute to this finding. Moreover, hepatic impairment was only indirectly assessed by the above-mentioned serum biomarkers, without any functional liver tests or liver histology. Nevertheless, the authors are highlighting an important point regarding hepatic function in critical illness, since it is well known that the liver is a key player in modulation of systemic inflammatory response and sepsis.
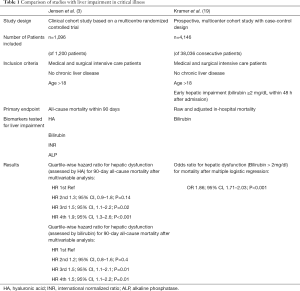
Full table
The liver in systemic inflammation and sepsis
The liver plays a fundamental role in immune surveillance and modulation of the immune response towards pathogens to either tolerance or pro-inflammatory response. The liver, receiving the blood from the gut via the portal vein, constitutes the second line of defence after the gut mucosa and its associated immune system. Since the liver also receives blood via the hepatic artery, it exerts central immunregulatory mechanisms in the response to blood-borne pathogens (21,22). The main site in the liver for interaction between invasive pathogens and cells involved in immune surveillance and immune response are the liver sinusoids, where Kupffer cells (i.e., the resident hepatic macrophages), endothelial cells, hepatic stellate cells and various immune cell subsets are located. The liver sinusoidal endothelial cells form a barrier separating the hepatocytes from the blood, and additionally act as sentinel and antigen-presenting cells. Equipped with a variety of immune receptors, co-stimulatory receptors, pattern recognition receptors and adhesion molecules they are able to recruit lymphocytes and form the immune platform for the variety of cells participating in the liver immune response (22). The liver sinusoids host neutrophils that react towards invading pathogens with phagocytosis and release of antimicrobial granule proteins. Further, neutrophils are able to release intranuclear DNA that form extracellular webs together with histones and proteases in order to trap and eliminate bacteria (so called neutrophil extracellular traps). On the other hand, this mechanism alters the sinusoidal blood flow and can contribute to ischemic liver injury (21). Hepatic macrophages including Kupffer cells and monocyte-derived macrophages can also directly kill bacteria via phagocytosis but also augment pro-inflammatory responses (23). Particularly Kupffer cells are equipped with scavenger receptors, toll-like receptors, complement receptors and antibody receptors allowing them to detect and internalize pathogens, which induces the release of pro-inflammatory cytokines like TNF-alpha, IL-6 and IL-1β and alerts and attracts immune cells (22,24). Pro-inflammatory cytokines trigger significant alterations in gene expression and metabolic functions of hepatocytes. This includes a downregulation of housekeeping genes precipitating in decreased metabolic function and bile formation, decreases iron mobilization into the blood and induces at the same time the synthesis and release of acute phase proteins. A large number of these acute phase proteins take active part in further activation of systemic immune response (21). The complexity of the interaction between resident liver cells and immune cells as well as the interactions between inflammatory cytokines, acute phase proteins and other humoral factors, shaping the systemic inflammatory responses, exceeds the mechanisms, as they are depicted here, by far. It is important to emphasize the central role of the liver in systemic inflammation as an endotoxin and bacterial scavenger, main organ for detoxification and producer of acute phase proteins and inflammatory cytokines. While these mechanisms substantially help in the host immune response, they also harbour the risk of inducing or aggravating hepatic injury in the case of overwhelming inflammation (24). Hypoxic hepatitis, for instance, is usually triggered by cardiac, circulatory or respiratory failure, but mechanisms of hepatic inflammation can worsen those effects. Recruitment of neutrophils and their adhesion at the liver sinusoidal cells as well as the production of neutrophil extracellular traps and subsequent thrombus formation can reduce the sinusoidal blood-flow and augment the harmful effects of hemodynamic alterations (21,24). Additionally, sinusoidal endothelial cells react to inflammatory signals via iNOS-dependent endothelial dysfunction with decreased vasodilatatory response and endothelin-1 (21), a vasoconstrictor increased in systemic inflammation, which induces contraction of liver sinusoids via activation of stellate cells (25).
Proinflammatory cytokines and endotoxins can alter the transcriptional and post-transcriptional gene expression of bile acid transporters and cause a downregulation of those transport proteins in hepatocytes. Therefore, in sepsis the excretion of bile acids into the bile ducts is impaired, leading to hepatocellular cholestasis (17,21,24). Cholangiocytes themselves are able to release proinflammatory signals (mainly TNF and IFNγ), which cause accumulation of immune cells and thereby periductular inflammation. Proinflammatory cytokines released within this inflammatory reaction impede the secretion of chloride and bicarbonate ions and therefore impair the bile flow, which leads to ductular cholestasis (17,21). Via the mechanisms of hepatocellular and ductular cholestasis, the condition of cholestatic liver dysfunction is directly linked to the immunologic processes that take place in the liver during systemic inflammation and sepsis.
Conclusions
There is emerging evidence for the outstanding impact of liver impairment on the prognosis in critically ill patients. However, the early detection of such dysfunction remains challenging. The detection of HA levels in critical illness as an additional parameter might be a promising approach to increase the sensitivity to detect mild liver dysfunction. Jensen et al. demonstrated that this impairment is especially important in sepsis, as the liver has a crucial role in the immune response towards systemic infection. However, the mechanism known so far rather suggest that sepsis itself is able to induce or augment liver injury in critical illness. In order to fully understand the mechanisms that cause the increase of mortality in the presence of liver impairment and to improve therapeutic strategies for those patients, further studies are needed.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Moseley RH. Sepsis and cholestasis. Clin Liver Dis 2004;8:83-94. [Crossref] [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Jensen JS, Peters L, Itenov TS, et al. Biomarker-assisted identification of sepsis-related acute liver impairment: a frequent and deadly condition in critically ill patients. Clin Chem Lab Med 2019;57:1422-31. [Crossref] [PubMed]
- Gibson PR, Fraser JRE, Brown TJ, et al. Hemodynamic and Liver Function Predictors of Serum Hyaluronan in Alcoholic Liver Disease. Hepatology 1992;15:1054-9. [Crossref] [PubMed]
- Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem 2016;49:302-15. [Crossref] [PubMed]
- Engström-Laurent A, Lööf L, Nyberg A, et al. Increased Serum Levels of Hyaluronate in Liver Disease. Hepatology 1985;5:638-42. [Crossref] [PubMed]
- Parés A, Deulofeu R, Giménez A, et al. Serum Hyaluronate Reflects Hepatic Fibrogenesis in Alcoholic Liver Disease and Is Useful as a Marker of Fibrosis. Hepatology 1996;24:1399-403. [Crossref] [PubMed]
- Plevris N, Sinha R, Way AW, et al. Index serum hyaluronic acid independently and accurately predicts mortality in patients with liver disease. Aliment Pharmacol Ther 2018;48:423-30. [Crossref] [PubMed]
- Rostved AA, Ostrowski SR, Peters L, et al. Hyaluronic Acid Is a Biomarker for Allograft Dysfunction and Predicts 1-Year Graft Loss After Liver Transplantation. Transplant Proc 2018;50:3635-43. [Crossref] [PubMed]
- Ugamura A, Chu P, Nakamoto N, et al. Liver Fibrosis Markers Improve Prediction of Outcome in Non-Acetaminophen-Associated Acute Liver Failure. Hepatol Commun 2018;2:1331-43. [Crossref] [PubMed]
- Yagmur E, Koch A, Haumann M, et al. Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin Biochem 2012;45:82-7. [Crossref] [PubMed]
- Bacher A, Zimpfer M. Hot Topics in Liver Intensive Care. Transplant Proc 2008;40:1179-82. [Crossref] [PubMed]
- Olson JC, Wendon JA, Kramer DJ, et al. Intensive Care of the Patient with Cirrhosis. Hepatology 2011;54:1864-72. [Crossref] [PubMed]
- Levesque E, Saliba F, Ichaï P, et al. Outcome of patients with cirrhosis requiring mechanical ventilation in ICU. J Hepatol 2014;60:570-8. [Crossref] [PubMed]
- Meersseman P, Langouche L, du Plessis J, et al. The intensive care unit course and outcome in acute-on-chronic liver failure are comparable to other populations. J Hepatol 2018;69:803-9. [Crossref] [PubMed]
- Fuhrmann V, Kneidinger N, Herkner H, et al. Hypoxic hepatitis: underlying conditions and risk factors for mortality in critically ill patients. Intensive Care Med 2009;35:1397-405. [Crossref] [PubMed]
- Jenniskens M, Langouche L, Vanwijngaerden Y, et al. Cholestatic liver (dys)function during sepsis and other critical illnesses. Intensive Care Med 2016;42:16-27. [Crossref] [PubMed]
- Brienza N, Dalfino L, Cinnella G, et al. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med 2006;32:267-74. [Crossref] [PubMed]
- Kramer L, Jordan B, Druml W, et al. Incidence and prognosis of early hepatic dysfunction in critically ill patients - A prospective multicenter study. Crit Care Med 2007;35:1099-104. [Crossref] [PubMed]
- Jensen JU, Hein L, Lundgren B, et al. Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med 2011;39:2048-58. [Crossref] [PubMed]
- Strnad P, Tacke F, Koch A, et al. Liver — guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol 2017;14:55-66. [Crossref] [PubMed]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol 2013;14:996-1006. [Crossref] [PubMed]
- Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol 2017;17:306-21. [Crossref] [PubMed]
- Nesseler N, Launey Y, Aninat C, et al. Clinical review: The liver in sepsis. Crit Care 2012;16:235. [Crossref] [PubMed]
- Sakamoto M, Ueno T, Kin M, et al. Ito cell contraction in response to endothelin-1 and substance P. Hepatology 1993;18:978-83. [Crossref] [PubMed]


