
Cystathionine gamma lyase aggravates orthodontic root resorption in mice
Introduction
Orthodontic tooth movement (OTM) is accompanied by periodontal tissue remodeling (1), which could improve masticatory ability and restore aesthetics (2,3). However, there are many risks associated with this procedure. Root resorption is one of familiar complications during orthodontic treatment. Previous studies showed that over 90% of orthodontic teeth would suffer from some degree of tooth resorption (4). It seems that root resorption is an unpredictable and unavoidable complication of orthodontic treatment (5). This undesirable result can reduce tooth stability, even cause permanent loss of the tooth. Various cells and cytokines are involved in the complicated process of orthodontic root resorption. Odontoclast with similar biological features to osteoclast (6,7) has been considered to be responsible for the process of orthodontic root resorption (8). Antibody against receptor of macrophage colony-stimulating factor (M-CSF) blocked orthodontically induced osteoclastogenesis, odontoclastogenesis and root resorption (9-11). Osteoprotegerin (OPG)/RANK/receptor activator of nuclear factor kappa-B ligand (RANKL) signaling pathway is known to play a crucial role in the differentiation of osteoclasts. OPG prevents RANK mediated activation of osteoclastic differentiation by binding to RANKL (12). Excessive orthodontic force induced bone resorption on the surface of the alveolar bone and increased OPG-positive cells and production of RANKL/RANK in osteoclasts and odontoclasts in the periodontal tissue, indicating OPG/RANK/RANKL might be involved in the process of root resorption, (13,14).
Cystathionine gamma lyase (CSE) is a synthetase for hydrogen sulfide (H2S), a gas transmitter produced by the somatic cell. H2S contributes to a variety of pathophysiological processes in vivo (15). In recent years, many studies have shown that CSE mediates osteoclast activities (16,17). Whether CSE-H2S modulates the root resorption by regulating osteoclasts and odontoclasts remains unclear. The purpose of this study was to investigate the effect of CSE on root resorption as well as on OPG/RANKL expression under physiological condition and during orthodontic treatment in mice. The data might provide a useful reference for the treatment and prevention of orthodontic root resorption in clinical practice.
Methods
Animals and OTM
All animal procedures were in accordance with the Animal Management Regulations of the Ministry of Health of the People’s Republic of China (Document No. 55, 2001) and approved by the Animal Use and Care Committee of Tongji University. CSE knock-out (CSE-/-) mice were bred at the Shanghai Biomodel Organisms Center, Inc. 8, 26 and 52 weeks old male wild-type and CSE-/- mice were used to evaluate physiological root resorption. Each group has five animals. The orthodontic animal model was established on 8-week-old male mice. Mice were housed under specific pathogen-free conditions with controlled temperature (22±1 °C), humidity (40–60%) and a 12-h light/dark cycle, and fed with soft food and water ad libitum.
To examine the effect of CSE on the orthodontic root resorption, an OTM mouse model was established as described in a previous study (15). Briefly, a nickel-titanium coiled spring (0.2 mm in thickness, 1 mm in diameter and 10 mm in length, Smart Technology Co, Ltd., Beijing, China) was placed between maxillary incisor and first molar for 1, 2 and 3 weeks. The force produced was approximately 0.35N (18) as measured by a dynamometer (HF-2; ALIYIOI). The right side without appliance was used as control.
Micro-CT scanning
The molars of mice were extracted from the maxillary bone carefully. The surrounding tissues were removed with tweezers and needles. The tooth was immersed in 2 percent sodium hypochlorite for about 20 min. Root resorption craters were evaluated by micro-CT (SCANCO Medical AG, Bruttisellen, Switzerland) after the surface of the root surface was completely clear. The maxillary first molars were reconstructed by a self-contained 3D analysis software of micro-CT (SCANCO Medical AG).
Electron microscope scanning
The molars were further examined with a scanning electron microscopy (SEM) (TM-1000; Hitachi, Tokyo, Japan). The ratio of the root resorption area was calculated using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA) (19). In addition, we assessed the root resorption by calculating the proportion of resorbed area on the pressure side of the distobuccal root of the maxillary first molar.
Histological analysis
Maxillae from all groups were dissected and prepared for histological analysis. The specimens were fixed in 4% paraformaldehyde at 4 °C for 24 h, followed with decalcification (4 °C for 4 weeks) by incubation with 10% ethylenediaminetetraacetic Acid (pH 7.4). The specimens were dehydrated in a series of alcohol baths beginning with 50% and progressing to 100%. Thereafter, the samples were embedded in paraffin and five 4-µm horizontal sections were obtained at a depth of 100, 140, 180, 220 and 260 µm from the first molar bifurcation surface. and stained with hematoxylin and eosin (HE) and titrate-resistant acid phosphatase (TRAP) staining. We assessed the root resorption by the proportion of resorbed area on the pressure side of the distobuccal root of the maxillary first molar.
RNA extraction and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from the alveolar bone surrounding the region of the first molar was extracted using TRIzol reagent (Invitrogen, USA) . RT-PCR Kit (Takara Bio, Inc., Otsu, Japan) was used to obtain complementary (c) DNA. Relative mRNA levels of OPG and RANKL were determined using a SYBR Green PCR Master mix and a LightCycler®96 Instrument (Roche, USA) following the manufacturer’s protocol. The sequences for the RT-PCR primers used in this study are as follows: OPG, 5'-GTGGAATAGATGTCACCCTGTGT- 3', 5'-TTTGGTCCCAGGCAAACTGT-3'; RANKL, 5'- CAGAAGATGGCACTCACTGCA-3', 5'-CACCATCGCTTTCTCTGCTCT-3'; GAPDH, 5'-AGCAGTCCCGTACACTGGCAAAC-3'’, 5'-TCTGTGGTGATGTAAATGTCCTCT-3' (Table 1).

Full table
Statistical analysis
Data were expressed as mean ± standard deviation. The statistical significance of differences among groups was calculated by independent 2-tailed Student’s t-tests or one-way analysis of variance (ANOVA). P<0.05 was considered significant.
Results
CSE deficiency-reduced physiological root resorption (Figure 1)
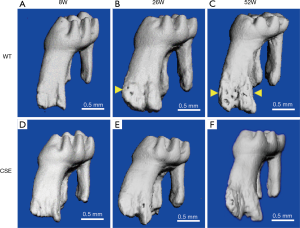
For the purpose of probing the function of CSE on physiological root resorption, we evaluated the root resorption in wide type (WT) and CSE-/- mice under physiological condition with micro-CT. Thirty (15 WT and 15 CSE-/-) mice at the age of 8, 26 and 52 weeks were used to examine the physiological root resorption of the maxillary first molar. We found that no root resorption occurred in 8 weeks old WT and CSE-/- mice (Figure 1A,D). Root resorption increased significantly in 26 weeks old mice (Figure 1B), and severe root resorption appeared at the age of 52 weeks in WT mice (Figure 1C). Root resorption was observed in 26 weeks old CSE-/- mice (Figure 1E), and became more obvious at the age of 52 weeks (Figure 1F). It suggested that the root resorption increased with age under physiological condition and the CSE deficiency decreased root resorption in the 26- and 52-week old animals compared with WT group.
CSE deficiency-reduced orthodontic root resorption assessed with micro-CT (Figure 2)
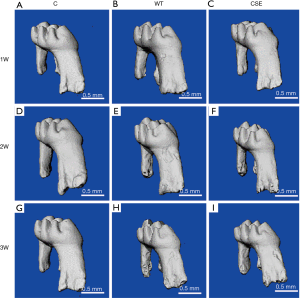
In order to look into the effect of CSE on the orthodontic root resorption, we used micro-CT to assess the distobuccal root of the maxillary first molar of WT and CSE-/- with 1-, 2- and 3-week orthodontic treatment. We found root resorption lacunae (RRL) after loading orthodontic force for 1 week (Figure 2B,C). Root resorption became more obvious after loading for 2 weeks (Figure 2E,F) and was significantly aggravated at the end of 3 weeks loading (Figure 2H,I). At the same time point, root resorption in WT group was severer than that in CSE-/- group. It suggested that the longer the orthodontic time, the more severe the root resorption is and knockout of the CSE gene can minimize the orthodontic root resorption.
CSE deficiency reduced orthodontic root resorption determined by SEM (Figure 3)
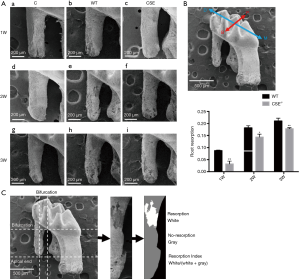
We also used the SEM to determine whether CSE deficiency altered the root resorption for maxillary first molar of the WT and CSE-/- mice following 1-, 2- and 3-week force application. We found that the SEM data showed a similar trend as the radiographic examinations above (Figure 3A). We further evaluated the root resorption index among the groups and found that the root resorption showed a trend of increase with orthodontic time, and the root resorption in CSE-/- mice was less than that in WT animals. The changes were statistically significant (Figure 3D).
CSE deficiency reduced orthodontic root resorption confirmed with HE staining (Figure 4)
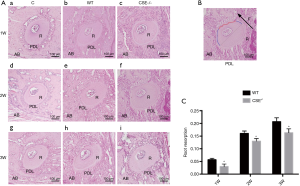
Five horizontal sections of the maxillary first molars from each WT and CSE-/- animal with orthodontic treatment for 1, 2, and 3 weeks were assessed for the distobuccal RRL (Figure 4A). The root resorption index showed a trend of enhanced root resorption with the increase of orthodontic time, and the root resorption in CSE-/- mice was less compared with WT group, which was statistically significant (Figure 4C).
CSE deficiency decreased orthodontically induced osteoclasts and mRNA expression of RANKL/OPG (Figure 5)
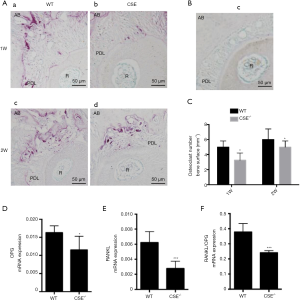
In this study, TRAP staining was performed to evaluate the role of CSE in root resorption and osteoclast response following 2-week orthodontic treatment. It was found CSE deficiency reduced orthodontic root resorption (Figure 5A) and osteoclasts. The changes were statistically significant (Figure 5C). The mRNA expression of RANKL/OPG in alveolar bone around the maxillary first molar was measured by RT-PCR (Figure 5D,E,F). The expression in CSE-/- group was lower compared with WT mice.
Discussion
Root resorption is a physiological or pathological phenomenon of cementum and dentin defects (20). The incidence of orthodontic root resorption is extremely high. Root resorption can lead to an imbalance in the ratio of crown and root, reduction in root length, poor tooth stability in long term, even loss of tooth (21). On the pressure side of OTM, osteoclasts activated by many molecules in periodontal tissue cause the resorption of bone. Odontoclasts in cementum, as part of periodontal tissue, is also affected. Odontoclasts are very similar to osteoclasts. Under pressure, odontoclasts are activated to cause cementum resorption, which is similar to the process of alveolar bone resorption caused by osteoclasts. This phenomenon is considered as the orthodontic root resorption (22).
In this study, we found that the root resorption of maxillary first molars in both CSE-/- and WT mice was aggravated with age, suggesting that age might be a risk factor of root resorption. The possible causes include a decreased periodontal vascularity and inelasticity, thicker cementum and its firm attachment in the apical third of the root with increased susceptibility (23), Our data showed that CSE-/- mice has less age-related resorption compared with WT mice. CSE is a key enzyme that controls production of H2S in the periodontal tissue. Our study indicates that CSE H2S might play a substantial role in physiological root resorption.
This study also demonstrated that the resorption area in both CSE-/- mice and WT mice increased with orthodontic time, indicating that the effects of orthodontics on root resorption is time-dependent. At each time point, the resorption area and the number of odontoclasts in CSE-/- group was less compared with the WT group, suggesting that CSE-H2S plays a significant role in orthodontically induced root resorption caused by odontoclasts.
There are many factors that affect root resorption, such as sex, age, genetics, systemic factors, tooth type, root shape, and previous medical history. In addition, orthodontic treatment duration, applied force size, movement type and extraction treatment are associated with orthodontically induced root resorption (24). In the clinical orthodontic practice, orthodontists attempt to find an “optimal” force to remove the dislocated tooth. However, root resorption seems to be inevitable even with light force (25). A previous study has found that 35g of orthodontic force can effectively move orthodontic teeth while minimizing tissue damage (26). However, we found that using 35g of force to move a mouse's maxillary first molar for a week resulted in root resorption.
During orthodontic treatment, the mechanical force causes periodontal ligament cells to secrete RANKL and OPG, affecting differentiation and activation of osteoclasts and odontoclasts, and leading to root resorption (9-11,27). RANKL/OPG is an important inducer of osteoclastogenesis. Activation of OPG blocks osteoclastogenesis and accelerates mature osteoclast apoptosis by inhibiting RANK bing to RANK (28). RANK and OPG mRNA expression increased in tissue during heavy force-induced root resorption (29). Our results showed that CSE deficiency reduced orthodontically induced osteoclasts, RANKL/OPG mRNA expression in surrounding alveolar of the maxillary first molar and root resorption. The number of osteoclasts is correlated with the degree of root resorption (data not shown). The data indicate that CSE may mediate root resorption by affecting odontoclasts. Growth hormone promoted heavy force-induced root resorption by up-regulating RANKL/OPG and IGF-1 in PDL cells at the early stage of tooth movement (14). OPG is osteoprotegerin that competitively binds to the RANK receptor RANKL site and inhibits the binding of RANK to RANKL, thereby inhibiting osteoclast activation (30). We examined the level of RANKL/OPG in surrounding periodontal tissue of the maxillary first molar in mice following 2-week orthodontic treatment. It was found that the number of osteoclast, the RANKL/OPG mRNA expression and root resorption in CSE-/- mice was lower than that in WT mice. Our previous studies have shown that orthodontic force treated CSE-/- mouse had less osteoclasts compared with WT group (16). The data in this study suggested that CSE can promote orthodontic root resorption by increasing osteoclast differentiation and regulating RANK/RANKL/OPG (31). Odontoclasts and osteoclasts resorb tooth and alveolar bone, respectively. However, the process of bone resorption is similar (32). Our study demonstrated that TRAP-positive cells existed not only on the root surface but also in the PDL tissues. Our results suggested that both osteoclasts and odontoclasts participated in the root resorption by a common mechanism, such as RANK/RANKL/OPG pathway or others (32).
Conclusions
In conclusion, the results of our study demonstrated that CSE deficiency reduced formation of osteoclasts and odontoblasts, which consequently downregulated physiological and orthodontic root resorption by disruption of the mechanisms of RANKL/OPG-induced osteoclastogenesis and odontoclastogenesis, suggesting careful planning and execution of orthodontic mechanics, such as considering age at the start of treatment and duration of treatment.
Acknowledgments
Funding: This study was supported by the National Science Foundation of China (No. 81870791) and the Shanghai Science and Technology Committee (No. 17140903700).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Animal Use and Care Committee of Tongji University (2018-003).
References
- Krishnan V, Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J Dent Res 2009;88:597-608. [Crossref] [PubMed]
- Henrikson T, Ekberg E, Nilner M. Can orthodontic treatment improve mastication? A controlled, prospective and longitudinal study. Swed Dent J 2009;33:59-65. [PubMed]
- Kikuta J, Yamaguchi M, Shimizu M, et al. Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells. J Dent Res 2015;94:140-7. [Crossref] [PubMed]
- Weltman B, Vig KW, Fields HW, et al. Root resorption associated with orthodontic tooth movement: a systematic review. Am J Orthod Dentofacial Orthop 2010;137:462-76; discussion 12A.
- Roscoe MG, Meira JB, Cattaneo PM. Association of orthodontic force system and root resorption: A systematic review. Am J Orthod Dentofacial Orthop 2015;147:610-26. [Crossref] [PubMed]
- Lossdörfer S, Götz W, Jäger A. Immunohistochemical localization of receptor activator of nuclear factor kappaB (RANK) and its ligand (RANKL) in human deciduous teeth. Calcif Tissue Int 2002;71:45-52. [Crossref] [PubMed]
- Sahara N, Toyoki A, Ashizawa Y, et al. Cytodifferentiation of the odontoclast prior to the shedding of human deciduous teeth: an ultrastructural and cytochemical study. Anat Rec 1996;244:33-49. [Crossref] [PubMed]
- Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res 2008;87:414-34. [Crossref] [PubMed]
- Kitaura H, Zhou P, Kim HJ, et al. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest 2005;115:3418-27. [Crossref] [PubMed]
- Kitaura H, Yoshimatsu M, Fujimura Y, et al. An anti-c-Fms antibody inhibits orthodontic tooth movement. J Dent Res 2008;87:396-400. [Crossref] [PubMed]
- Kitaura H, Fujimura Y, Yoshimatsu M, et al. An M-CSF receptor c-Fms antibody inhibits mechanical stress-induced root resorption during orthodontic tooth movement in mice. Angle Orthod 2009;79:835-41. [Crossref] [PubMed]
- Yang PT, Kasai H, Xiao WG, et al. Increased expression of macrophage colony-stimulating factor in ankylosing spondylitis and rheumatoid arthritis. Ann Rheum Dis 2006;65:1671-2. [Crossref] [PubMed]
- Nakano Y, Yamaguchi M, Fujita S, et al. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod 2011;33:335-43. [Crossref] [PubMed]
- Hu Y, Liu W, Liu Z, et al. Receptor activator of nuclear factor-kappa ligand, OPG, and IGF-I expression during orthodontically induced inflammatory root resorption in the recombinant human growth hormone-treated rats. Angle Orthod 2015;85:562-9. [Crossref] [PubMed]
- Pu H, Hua Y. Hydrogen sulfide regulates bone remodeling and promotes orthodontic tooth movement. Mol Med Rep 2017;16:9415-22. [Crossref] [PubMed]
- Mo S, Hua Y. Cystathionine gamma lyase-H2S contributes to osteoclastogenesis during bone remodeling induced by mechanical loading. Biochem Biophys Res Commun 2018;501:471-7. [Crossref] [PubMed]
- Zheng Y, Liao F, Lin X, et al. Cystathionine gamma-Lyase-Hydrogen Sulfide Induces Runt-Related Transcription Factor 2 Sulfhydration, Thereby Increasing Osteoblast Activity to Promote Bone Fracture Healing. Antioxid Redox Signal 2017;27:742-53. [Crossref] [PubMed]
- Irie K, Ekuni D, Yamamoto T, et al. A single application of hydrogen sulphide induces a transient osteoclast differentiation with RANKL expression in the rat model. Arch Oral Biol 2009;54:723-9. [Crossref] [PubMed]
- Fujimura Y, Kitaura H, Yoshimatsu M, et al. Influence of bisphosphonates on orthodontic tooth movement in mice. Eur J Orthod 2009;31:572-7. [Crossref] [PubMed]
- Pizzo G, Licata ME, Guiglia R, et al. Root resorption and orthodontic treatment. Review of the literature. Minerva Stomatol 2007;56:31-44. [PubMed]
- Viecilli RF, Kar-Kuri MH, Varriale J, et al. Effects of initial stresses and time on orthodontic external root resorption. J Dent Res 2013;92:346-51. [Crossref] [PubMed]
- Chung CJ, Soma K, Rittling SR, et al. OPN deficiency suppresses appearance of odontoclastic cells and resorption of the tooth root induced by experimental force application. J Cell Physiol 2008;214:614-20. [Crossref] [PubMed]
- Krishnan V. Root Resorption with Orthodontic Mechanics: Pertinent Areas Revisited. Aust Dent J 2017;62 Suppl 1:71-7. [Crossref] [PubMed]
- Samandara A, Papageorgiou SN, Ioannidou-Marathiotou I, et al. Evaluation of orthodontically induced external root resorption following orthodontic treatment using cone beam computed tomography (CBCT): a systematic review and meta-analysis. Eur J Orthod 2019;41:67-79. [Crossref] [PubMed]
- Wishney M. Potential risks of orthodontic therapy: a critical review and conceptual framework. Aust Dent J 2017;62 Suppl 1:86-96. [Crossref] [PubMed]
- Taddei SR, Moura AP, Andrade I Jr, et al. Experimental model of tooth movement in mice: a standardized protocol for studying bone remodeling under compression and tensile strains. J Biomech 2012;45:2729-35. [Crossref] [PubMed]
- Matsuda Y, Motokawa M, Kaku M, et al. RANKL and OPG expression: Jiggling force affects root resorption in rats. Angle Orthod 2017;87:41-8. [Crossref] [PubMed]
- Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 2008;473:139-46. [Crossref] [PubMed]
- Low E, Zoellner H, Kharbanda OP, et al. Expression of mRNA for osteoprotegerin and receptor activator of nuclear factor kappa beta ligand (RANKL) during root resorption induced by the application of heavy orthodontic forces on rat molars. Am J Orthod Dentofacial Orthop 2005;128:497-503. [Crossref] [PubMed]
- Baud'huin M, Duplomb L, Teletchea S, et al. Osteoprotegerin: multiple partners for multiple functions. Cytokine Growth Factor Rev 2013;24:401-9. [Crossref] [PubMed]
- Kurabayashi M. Hydrogen sulfide: a new regulator of osteoclastogenesis? Arterioscler Thromb Vasc Biol 2014;34:471-3. [Crossref] [PubMed]
- Hakami Z, Kitaura H, Kimura K, et al. Effect of interleukin-4 on orthodontic tooth movement and associated root resorption. Eur J Orthod 2015;37:87-94. [Crossref] [PubMed]


