
Orexin-A aggravates cytotoxicity and mitochondrial impairment in SH-SY5Y cells transfected with APPswe via p38 MAPK pathway
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease. The prevalence of AD is growing at a fast rate with the increase in life expectancy (1). Extracellular amyloid plaques (deposits of Aβ peptide) and intracellular neurofibrillary tangles (accumulation of hyperphosphorylated tau protein) are the main pathological hallmarks of AD (2). Many studies have focused on the neurotoxicity of Aβ (3,4). Aβ, including Aβ1–40 and Aβ1–42, derives from amyloid precursor protein (APP) through amyloidogenic and non-amyloidogenic pathways (5).
Orexin, also known as hypocretin, is produced in the hypothalamus and includes Orexin-A and Orexin-B (6). The orexinergic neurons project into several brain areas (7). By activating its receptor, Orexin-A participates in many physiological processes, such as sleep/wake regulation, modulation of energy metabolism, feeding, learning, and memory (8-11). Many researchers believe that Orexin-A is involved in the pathogenesis of AD (12,13). The overexpression of Orexinergic signaling alters the sleep-wake cycle, and induces the accumulation of Aβ and neurodegeneration mediated by tau protein (12). A higher concentration of Orexin-A was found in moderate to severe AD patients than in controls (14). Furthermore, the level of Orexin-A in cerebral spinal fluid (CSF) increased in mild cognitive impairment (MCI) patients due to AD compared with controls (15).
Mitochondria are responsible for energy production in cells and are vital for cell survival. Mitochondrial dysfunction is a critical mechanism for the progression of AD, which occurs in the early phase of AD and worsens with its severity (16,17). Orexin-A has been shown to regulate mitochondrial function and dynamics (18). Therefore, it is very important to investigate the change of mitochondria induced by Orexin-A in AD. The p38 MAPK pathway is widely expressed in the central nervous system and activated in AD (19,20), which promotes the phosphorylation of tau protein and plays a crucial role in cell death and apoptosis (21-23). Orexin-A was reported to activate the p38 MAPK pathway and induce cell death via the p38 MAPK pathway (24). Therefore, we sought to explore whether the effect of Orexin-A on AD was mediated through the p38 MAPK pathway.
In this study, we used SH-SY5Y cells that were stably transfected with Swedish mutant amyloid precursor protein (APPswe), a cell model of AD that has been extensively used by researchers (25-28). We sought to investigate the effect of Orexin-A on APPswe cells, and discover the underlying mechanism and signaling pathway.
Methods
Materials and cell culture
Orexin-A and the p38 MAPK inhibitor SB203580 were purchased from Sigma-Aldrich (Missouri, USA). Human neuroblastoma SH-SY5Y cells stably transfected with empty vector (empty vector cells) or vector carrying APPswe (APPswe cells) were generated using Lipofectamine 2000 (Invitrogen, California, USA). The transfected cells were selected using puromycin, and cultured in DMEM culture medium with 10% fetal bovine serum and 1% streptomycin-penicillin in an incubator with 5% CO2 at 37 °C.
Western blotting
Cell were homogenized in RIPA lysis buffer containing 1% protease inhibitors. The homogenates were centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants were used. After measuring the protein concentration using the BCA assay kit (Thermo Scientific, Massachusetts, USA), protein samples were added with loading buffer, and boiled for 5 min at 95 °C to denature proteins. Equal amounts of protein samples were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membrane. The membrane was blocked using a blocking buffer and incubated with primary antibody against APP (Abcam, 1:20,000), total p38 (t-p38) (CST, 1:1,000), phospho-p38 (p-p38) (CST, 1:1,000) or β-actin (CST, 1:1,000) overnight at 4 °C, followed by incubation with goat anti-rabbit IgG secondary antibody (Proteintech, 1:5,000) for 1 h at room temperature. After visualized by an ECL chemiluminescence kit (Millipore, Massachusetts, USA) and a chemiluminescence imaging system (Bio-Rad, California, USA), bands were quantified using Image J software.
Measurement of Aβ1–40 and Aβ1–42
Cell medium was collected and centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatants were collected to measure the amount of Aβ1–40 and Aβ1–42 by an ELISA assay kit (Invitrogen, California, USA) following the manufacturer’s instructions.
Cell viability assay
According to the protocol described in the CCK-8 assay kit (Dojindo, Kumamoto, Japan), cells were planted in 96-well plates. Following various treatments, cells were added with CCK-8 and were incubated at 37 °C for 4 h. The absorbance was read at 450 nm with a microplate reader (Biotek, Vermont, USA).
Measurement of cell proliferation
Cells were seeded in 96-well plates and serum-deprived for 24 h. BrdU was added and cells were incubated for 4 h. The BrdU incorporation into the DNA of dividing cells was measured using the ELISA BrdU assay kit (Abcam, Cambridge, UK).
Electron microscopy
Cells were fixed in 2.5% glutaraldehyde overnight, followed by fixation with osmium for 1 h. Subsequently, cells were embedded in epoxy resin. Samples were cut using an ultramicrotome (Leica, Wetzlar, Germany), and stained with uranyl acetate and lead citrate. Electron microscopy (Hitachi, Tokyo, Japan) was used to capture images of mitochondria.
Measurement of ATP
ATP level was determined using the ATP bioluminescence assay kit (Roche, Basal, Switzerland). Cells were homogenized in lysis buffer on ice and centrifuged at 12,000 rpm for 15 min at 4 °C. ATP level in supernatants was measured using a luminescence plate reader (Biotek, Vermont, USA).
Detection of mitochondrial DNA (mtDNA) copy number
The mtDNA copy number, measured by the number of mitochondrial genome per nuclear genome, is a useful marker of mitochondrial dysfunction (29). The mtDNA copy number was measured using real-time PCR. The total intracellular DNA was extracted from cells using the QIAamp DNA mini kit (Qiagen, California, USA) according to manufacturer’s instructions. Each real-time PCR reaction consisted of 1 µL each of the forward and reverse primers, 10 µL of 2× SYBR green real-time PCR master (Applied Biosystems, Massachusetts, USA), 3 µL of template DNA, and 5 µL of ultrapure water. The primers for human electron transport chain, which were used for mtDNA amplification, were as follows: forward, 5'-CAAACCTACGCCAAAATCCA-3' and reverse, 5'-GAAATGAATGAGCCTACAGA-3'. The premiers for human nuclear 18s (18s RNA), which were used for standardization, were as follows: forward, 5'-ACGGACCAGAGCGAAAGCA-3', and reverse, 5'-GACATCTAAGGGCATCACAGAC-3'. The mtDNA copy number was calculated using the 2−ΔΔCt method (30).
Isolation of mitochondria
Cells were washed with PBS, harvested, and pooled. Cells were homogenized in lysis buffer for 15 min at 4 °C. The homogenates were spun at 800 g for 5 min, and supernatants were centrifuged at 15,000 g for 10 min at 4 °C to obtain mitochondrial pellets. The pellets were then resuspended in lysis buffer.
Measurement of cytochrome C oxidase (CCO)
The activity of CCO was measured using the CCO assay kit (Sigma Aldrich, Missouri, USA). The mitochondrial fractions were added to the enzyme dilution buffer. The reaction was started by addition of the ferrocytochrome c substrate solution. The change in absorbance at 550 nm was recorded with a 5 s delay and 10 s interval for 6 readings on a microplate reader (Biotek, Vermont, USA).
Measurement of reactive oxygen species (ROS)
According to the protocol of the ROS assay kit (Sigma Aldrich, Missouri, USA), the intracellular level of ROS was measured using the fluorescence dye 2’,7’-dichlorodihydrofluorescein diacetate (H2DCFDA). Cells were seeded in 24-well plates, and incubated with H2DCFDA for 20 min at 37 °C. The fluorescence image was captured (Ex =485 nm, Em =525 nm) using fluorescence microscope (Olympus, Tokyo, Japan), and the level of ROS, which was proportional to the fluorescence intensity of H2DCFDA, was quantified using Image J software.
Statistical analysis
SPSS 17.0 was used to perform statistical analysis. Each experiment was repeated in triplicates. Data were expressed as mean ± standard error of the mean (SEM). Difference between groups was determined by one-way or two-way analysis of variance (ANOVA), followed by post hoc Tukey’s multiple comparison. Chi-squared test with Bonfferoni’s correction was used to analyze mitochondria in electron microscopy images. Statistical significance was set at P<0.05.
Results
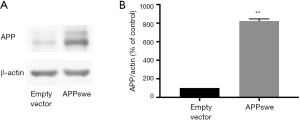
APP level increased in APPswe cells
Firstly, we analyzed the expression of APP using western blotting assay. As shown in Figure 1, there was higher expression of APP in APPswe cells compared with empty vector cells (P<0.01).
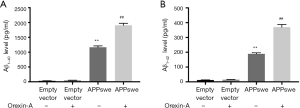
Orexin-A increased the level of Aβ1–40 and Aβ1–42 in APPswe cells
Next, we tested the level of Aβ1–40 and Aβ1–42 in the cell medium using an ELISA kit. Cells were treated with 100 nM Orexin-A for 24 h. As shown in Figure 2, the level of Aβ1–40 and Aβ1–42 were significantly higher in APPswe cells compared with empty vector cells (P<0.01; P<0.01). When APPswe cells were treated with Orexin-A, the level of Aβ1–40 and Aβ1–42 increased (P<0.01; P<0.01). However, Orexin-A did not increase the expression of Aβ1–40 and Aβ1–42 in empty vector cells (P=0.9975; P=0.9838).
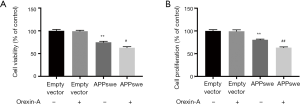
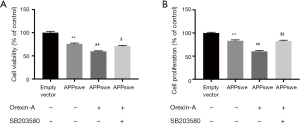
Orexin-A decreased cell viability and cell proliferation in APPswe cells
Cells were treated with 100 nM Orexin-A for 24 h, and cell viability and cell proliferation were analyzed. The result of the CCK-8 assay showed that cell viability of APPswe cells was lower than that of empty vector cells (P<0.01). Following treatment with Orexin-A, the cell viability of APPswe cells decreased even further (P<0.05). However, the cell viability of empty vector cells did not change after treatment with Orexin-A (P=0.9950) (Figure 3A). Cell proliferation was analyzed using the ELISA BrdU assay. APPswe cells showed reduced cell proliferation compared with empty vector cells (P<0.01). While the addition of Orexin-A had no significant effect on empty vector cells (P=0.9960), it reduced cell proliferation in APPswe cells further (P<0.01) (Figure 3B).
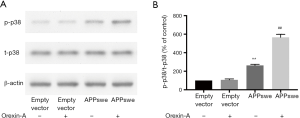
Orexin-A activated p38 MAPK pathway in APPswe cells
Next, we sought to identify the downstream pathways that mediate these changes. The p38 MAPK pathway is one of the MAPK pathways that has been reported to be activated by Orexin-A and related to cell death (24). Therefore, we want to determine whether Orexin-A could activate p38 MAPK pathway in APPswe cells. Cells were treated with Orexin-A for 3 h. The activation of p38 MAPK pathway was assessed by measuring the phosphorylation of p38 (expression ratio of p-p38 to t-p38). Phosphorylation of p38 increased in APPswe cells compared with empty vector cells (P<0.01). Following treatment with Orexin-A, the phosphorylation of p38 increased in APPswe cells (P<0.01), but not in empty vector cells (P=0.9935) (Figure 4).
Orexin-A decreased cell viability and cell proliferation via p38 MAPK pathway in APPswe cells
Given that the p38 MAPK pathway is activated by Orexin-A treatment and associated with cell death, we sought to determine whether the deleterious effect of Orexin-A on APPswe cells was mediated via the p38 MAPK pathway. Cells were pretreated with or without the p38 MAPK inhibitor SB203580 (10 µM) for 30 min, followed by treatment with 100 nM Orexin-A for 24 h. Orexin-A decreased the cell viability of APPswe cells as proved by the CCK-8 test (P<0.01), which was reversed by treatment with SB203580 (P<0.05) (Figure 5A). In the ELISA BrdU test, results demonstrated that Orexin-A decreased the cell proliferation of APPswe cells (P<0.01), which was also reversed by treatment with SB203580 (P<0.01) (Figure 5B).
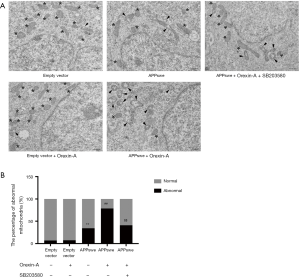
Orexin-A aggravated the impairment of mitochondrial morphology in APPswe cells, which was mediated by p38 MAPK pathway
To test whether Orexin-A had any deleterious effect on mitochondria, the mitochondrial morphology was observed by electron microscopy. Cells were pretreated with or without 10 µM SB203580 for 30 min, followed by treatment with 100 nM Orexin-A for 24 h. Representative electron microscopic images of mitochondria are shown in Figure 6A. In empty vector cells, most of mitochondria were normal, with or without Orexin-A treatment (Figure 6A, asterisk). In APPswe cells, however, abnormal mitochondria began to increase (Figure 6A, arrowhead), with vacuolar structure, severe swelling, or broken cristae. Following treatment with Orexin-A, most of mitochondria in APPswe cells were abnormal (Figure 6A, arrowhead). SB203580 blocked the impairment of mitochondrial morphology induced by Orexin-A in APPswe cells. The percentage of abnormal mitochondria was calculated and shown in Figure 6B. Compared with empty vector cells, the percentage of abnormal mitochondria was higher in APPswe cells (P<0.01). Orexin-A significantly increased the percentage of abnormal mitochondria in APPswe cells (P<0.01), while had no significant effect in empty vector cells (P=0.7383). Treatment with SB203580 reduced the percentage of abnormal mitochondria in Orexin-A treated APPswe cells (P<0.01).
Orexin-A aggravated mitochondrial dysfunction via p38 MAPK pathway in APPswe cells
To investigate the effect of Orexin-A on mitochondrial function, we tested CCO activity, ATP level, and mtDNA copy number. Cells were pretreated with or without 10 µM SB203580 for 30 min, followed by treatment with Orexin-A for 24 h. Mitochondrial respiratory function was assessed by measuring the activity of CCO, a key enzyme in mitochondrial respiratory chain. As shown in Figure 7A, compared with empty vector cells, APPswe cells showed decreased CCO activity (P<0.01). Orexin-A reduced CCO activity in APPswe cells (P<0.01), which was inhibited by treatment with SB203580 (P<0.05). Orexin-A did not have significant effect in empty vector cells (P=0.3511).

The ATP level, which reflects mitochondrial bioenergy, was also decreased in APPswe cells compared with empty vector cells (P<0.01). The ATP level showed a significant decline after treatment with Orexin-A in APPswe cells (P<0.01), but did not change markedly in empty vector cells (P=0.5748). SB203580 increased ATP level in Orexin-A treated APPswe cells (P<0.01) (Figure 7B).
The mtDNA copy number was lower in APPswe cells than empty vector cells (P<0.01). Orexin-A treatment decreased the mtDNA copy number in APPswe cells (P<0.01), but had no significant effect on empty vector cells (P=0.9978). Treatment with SB203580 increased the mtDNA copy number in Orexin-A treated APPswe cells (P<0.01) (Figure 7C).
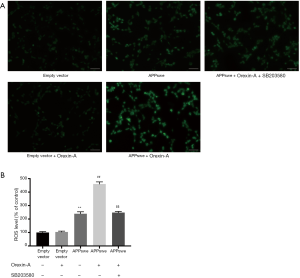
Orexin-A increased ROS level in APPswe cells through p38 MAPK pathway
Cells were pretreated with or without 10 µM SB203580 for 30 min, followed by treatment with Orexin-A for 24 h. Representative fluorescence images of ROS are shown in Figure 8A. APPswe cells showed increased level of ROS than empty vector cells (P<0.01), and Orexin-A treatment further increased the ROS level in APPswe cells (P<0.01), while had no significant effect in empty vector cells (P=0.9989). Treatment with SB203580 blocked the increase in ROS level induced by Orexin-A in APPswe cells (P<0.01) (Figure 8B).
Discussion
Orexin-A has been reported to participate in the pathogenesis of AD (12,13). In this study, we sought to determine the role of Orexin-A in AD. We report for the first time that Orexin-A aggravates cell death and mitochondrial impairment in SH-SY5Y cells transfected with APPswe. We further demonstrated that the deleterious effect of Orexin-A is mediated through p38 MAPK pathway.
Aβ, one of the most notable pathological hallmark of AD, is derived from APP through amyloidogenic and non-amyloidogenic pathways (5). APPswe cells showed increased level of APP, Aβ1–40, and Aβ1–42 compared with empty vector cells (Figures 1,2), indicating that it is a successful cell model of AD. Intracerebroventricular injection of Orexin-A increased the brain interstitial fluid (ISF) level of Aβ in APP/PS1 mice (31). When Orexin gene was knocked out in APP/PS1 mice, there was a marked decrease in the amount of Aβ pathology (32). In this study, we found that Orexin-A increased the level of Aβ1–40 and Aβ1–42 in APPswe cells (Figure 2), which is in line with previous studies and demonstrates the in vitro effect of Orexin-A. The aggravation of cell death and mitochondrial impairment caused by Orexin-A in APPswe cells may be due to the increase in Aβ level.
Cell death is one of the most common manifestations in AD (33-35). We found that APPswe cells showed reduced cell viability and cell proliferation. In previous studies, the role of Orexin-A in cell growth has been both positive and negative. Some studies have demonstrated a protective effect for Orexin-A (36,37), while others have shown its harmful effect (24,38,39) on cells. In this study, we found that Orexin-A decreased cell viability and cell proliferation in APPswe cells (Figure 3). The role of Orexin-A depends on the cell type and related disease, and its function may be complex and complicated. In our study, Orexin-A had a cytotoxic effect on APPswe cells, probably because Orexin-A is related to the pathogenesis of AD.
Mitochondria provide energy for cells, which is indispensable for cellular function. Mitochondrial impairment is a critical mechanism that causes inflammation and cell death (40). Mitochondrial impairment is an early step in AD, and it is observed in AD transgenic mice and cell lines that stably express APP (41-43). The accumulation of Aβ in mitochondria is associated with reduction of respiratory control rate (RCR), CCO activity, ATP level, dysregulation of mitochondrial energy metabolism, decrease in mitochondrial membrane potential (MMP), and increase in ROS (44-47). The accumulation of Aβ can also lead to impaired mitochondrial transport and mitochondrial dynamics (48). As evidenced by electron microscopy, mtDNA copy number, CCO activity, and ATP level, we found mitochondrial impairment in APPswe cells. We also found that Orexin-A caused severe damage to mitochondria in APPswe cells (Figures 6,7), suggesting that mitochondrial impairment is an important mechanism for the cytotoxic effect of Orexin-A on APPswe cells. ROS are comprised of free and nonfree radical oxygen, which contain molecules such as hydrogen peroxide (H2O2), superoxide (O2·−) and hydroxyl radical (·OH) (49,50). Mitochondria are the major source of endogenous ROS, and the dysfunction of mitochondria play an important role in ROS production (50,51). Our results showed that Orexin-A increased ROS level in APPswe cells (Figure 8), which can also reflect that Orexin-A cause mitochondrial damage in AD.
We further investigated the regulation of signaling pathway following treatment with Orexin-A. Our results showed that phosphorylation of p38 increased dramatically in APPswe cells compared with empty vector cells (Figure 4), suggesting that the p38 MAPK pathway correlates with the progression of AD. Because Orexin-A is reported to activate p38 MAPK pathway and induce cell death via p38 MAPK pathway (24), we hypothesized that Orexin-A aggravates the severity in APPswe cells by inducing p38 MAPK activation. Following treatment with Orexin-A, we found a further increase in the phosphorylation of p38 in APPswe cells (Figure 4). Orexin-A decreased cell viability and cell proliferation in APPswe cells, which was blocked by the p38 MAPK inhibitor SB203580 (Figure 5). These results indicate that Orexin-A modulates p38 MAPK pathway to produce cytotoxic effect in APPswe cells. SB203580 could also reverse the mitochondrial impairment induced by Orexin-A in APPswe cells (Figures 6-8), indicating that the deleterious effect of Orexin-A on mitochondria is also mediated through p38 MAPK pathway. The p38 MAPK pathway may be an important modulator of the effect of Orexin-A on APPswe cells.
In conclusion, we found that Orexin-A aggravated Aβ level, cytotoxicity, and mitochondrial impairment in SH-SY5Y cells transfected with APPswe. We also found that Orexin-A activated the p38 MAPK pathway, which could modulate the deleterious effect of Orexin-A in SH-SY5Y cells transfected with APPswe. Further studies are needed to explore the antagonists of Orexin-A receptor that may be effective in alleviating AD.
Acknowledgments
We thank all of the authors and staffs that contribute to this work.
Funding: This work was supported by the National Natural Science Foundation of China (81571052, 81870848), the Fundamental Research Funds of Chinese Academy of Medical Sciences (2019-RC-HL-026), Shandong provincial key research and development program (2017GSF218036), and the Fundamental Research Funds of Shandong University (2016JC022).
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All of the procedures described in this study were in accordance with the ethical provisions of Helsinki Declaration, and approved by the Ethics Committee of Shandong University.
References
- Swomley AM, Forster S, Keeney JT, et al. Abeta, oxidative stress in Alzheimer disease: evidence based on proteomics studies. Biochim Biophys Acta 2014;1842:1248-57. [Crossref] [PubMed]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell 2012;148:1204-22. [Crossref] [PubMed]
- Goate A, Hardy J. Twenty years of Alzheimer's disease-causing mutations. J Neurochem 2012;120 Suppl 1:3-8. [Crossref] [PubMed]
- Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med 2011;3:77sr1. [Crossref] [PubMed]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med 2010;362:329-44. [Crossref] [PubMed]
- de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 1998;95:322-7. [Crossref] [PubMed]
- Baldo BA, Daniel RA, Berridge CW, et al. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 2003;464:220-37. [Crossref] [PubMed]
- Aou S, Li XL, Li AJ, et al. Orexin-A (hypocretin-1) impairs Morris water maze performance and CA1-Schaffer collateral long-term potentiation in rats. Neuroscience 2003;119:1221-8. [Crossref] [PubMed]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 2013;7:28. [Crossref] [PubMed]
- Girault EM, Yi CX, Fliers E, et al. Orexins, feeding, and energy balance. Prog Brain Res 2012;198:47-64. [Crossref] [PubMed]
- Tsunematsu T, Tabuchi S, Tanaka KF, et al. Long-lasting silencing of orexin/hypocretin neurons using archaerhodopsin induces slow-wave sleep in mice. Behav Brain Res 2013;255:64-74. [Crossref] [PubMed]
- Liguori C. Orexin and Alzheimer's Disease. Curr Top Behav Neurosci 2017;33:305-22. [Crossref] [PubMed]
- Ferini-Strambi L. Possible role of orexin in the pathogenesis of Alzheimer disease. JAMA Neurol 2014;71:1478-80. [Crossref] [PubMed]
- Liguori C, Romigi A, Nuccetelli M, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 2014;71:1498-505. [Crossref] [PubMed]
- Liguori C, Nuccetelli M, Izzi F, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer's disease. Neurobiol Aging 2016;40:120-6. [Crossref] [PubMed]
- Hauptmann S, Scherping I, Drose S, et al. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging 2009;30:1574-86. [Crossref] [PubMed]
- de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J Alzheimers Dis 2006;9:167-81. [Crossref] [PubMed]
- Lassiter K, Greene E, Piekarski A, et al. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am J Physiol Regul Integr Comp Physiol 2015;308:R173-87. [Crossref] [PubMed]
- He XL, Yan N, Chen XS, et al. Hydrogen sulfide down-regulates BACE1 and PS1 via activating PI3K/Akt pathway in the brain of APP/PS1 transgenic mouse. Pharmacol Rep 2016;68:975-82. [Crossref] [PubMed]
- Yin K, Jin J, Zhu X, et al. CART modulates beta-amyloid metabolism-associated enzymes and attenuates memory deficits in APP/PS1 mice. Neurol Res 2017;39:885-94. [Crossref] [PubMed]
- Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010;1802:396-405. [Crossref] [PubMed]
- Corrêa SA, Eales KL. The Role of p38 MAPK and Its Substrates in Neuronal Plasticity and Neurodegenerative Disease. J Signal Transduct 2012;2012:649079. [Crossref] [PubMed]
- Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer's disease. Neuropharmacology 2010;58:561-8. [Crossref] [PubMed]
- Ammoun S, Lindholm D, Wootz H, et al. G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem 2006;281:834-42. [Crossref] [PubMed]
- Chan KH, Lam KS, Cheng OY, et al. Adiponectin is protective against oxidative stress induced cytotoxicity in amyloid-beta neurotoxicity. PLoS One 2012;7:e52354. [Crossref] [PubMed]
- Bussiere R, Lacampagne A, Reiken S, et al. Amyloid beta production is regulated by beta2-adrenergic signaling-mediated post-translational modifications of the ryanodine receptor. J Biol Chem 2017;292:10153-68. [Crossref] [PubMed]
- Wang CY, Zheng W, Wang T, et al. Huperzine A activates Wnt/beta-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 2011;36:1073-89. [Crossref] [PubMed]
- Oulès B, Del Prete D, Greco B, et al. Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 2012;32:11820-34. [Crossref] [PubMed]
- Alvarez-Mora MI, Podlesniy P, Gelpi E, et al. Fragile X-associated tremor/ataxia syndrome: Regional decrease of mitochondrial DNA copy number relates to clinical manifestations. Genes Brain Behav 2019;18:e12565. [Crossref] [PubMed]
- Schefe JH, Lehmann KE, Buschmann IR, et al. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s C T difference” formula. J Mol Med (Berl) 2006;84:901-10. [Crossref] [PubMed]
- Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005-7. [Crossref] [PubMed]
- Roh JH, Jiang H, Finn MB, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med 2014;211:2487-96. [Crossref] [PubMed]
- Yu W, Mechawar N, Krantic S, et al. Evidence for the involvement of apoptosis-inducing factor-mediated caspase-independent neuronal death in Alzheimer disease. Am J Pathol 2010;176:2209-18. [Crossref] [PubMed]
- Lee JH, Cheon YH, Woo RS, et al. Evidence of early involvement of apoptosis inducing factor-induced neuronal death in Alzheimer brain. Anat Cell Biol 2012;45:26-37. [Crossref] [PubMed]
- Eckert A, Marques CA, Keil U, et al. Increased apoptotic cell death in sporadic and genetic Alzheimer's disease. Ann N Y Acad Sci 2003;1010:604-9. [Crossref] [PubMed]
- Pasban-Aliabadi H, Esmaeili-Mahani S, Abbasnejad M. Orexin-A Protects Human Neuroblastoma SH-SY5Y Cells Against 6-Hydroxydopamine-Induced Neurotoxicity: Involvement of PKC and PI3K Signaling Pathways. Rejuvenation Res 2017;20:125-33. [Crossref] [PubMed]
- Ju SJ, Zhao Y, Chang X, et al. Orexin A protects cells from apoptosis by regulating FoxO1 and mTORC1 through the OX1R/PI3K/AKT signaling pathway in hepatocytes. Int J Mol Med 2014;34:153-9. [Crossref] [PubMed]
- Biegańska K, Sokolowska P, Johren O, et al. Orexin A suppresses the growth of rat C6 glioma cells via a caspase-dependent mechanism. J Mol Neurosci 2012;48:706-12. [Crossref] [PubMed]
- Li G, Tang S, Chi H, et al. Orexin-A aggravates the impairment of hippocampal neurons caused by intermittent hypoxemia by the OXR-PLCbeta1-ERK1/2 pathway. Neuroreport 2017;28:331-8. [Crossref] [PubMed]
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011;333:1109-12. [Crossref] [PubMed]
- Leuner K, Schütt T, Kurz C, et al. Mitochondrion-Derived Reactive Oxygen Species Lead to Enhanced Amyloid Beta Formation. Antioxid Redox Signal 2012;16:1421-33. [Crossref] [PubMed]
- Xu YJ, Mei Y, Qu ZL, et al. Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction. Biomed Res Int 2018;2018:4606752. [Crossref] [PubMed]
- Fang D, Zhang Z, Li H, et al. Increased Electron Paramagnetic Resonance Signal Correlates with Mitochondrial Dysfunction and Oxidative Stress in an Alzheimer's disease Mouse Brain. J Alzheimers Dis 2016;51:571-80. [Crossref] [PubMed]
- Manczak M, Anekonda TS, Henson E, et al. Mitochondria are a direct site of A beta accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet 2006;15:1437-49. [Crossref] [PubMed]
- Du H, Guo L, Fang F, et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 2008;14:1097-105. [Crossref] [PubMed]
- Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer's disease. J Alzheimers Dis 2010;20 Suppl 2:S569-78. [Crossref] [PubMed]
- Caspersen C, Wang N, Yao J, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. FASEB J 2005;19:2040-1. [Crossref] [PubMed]
- Guo L, Du H, Yan S, et al. Cyclophilin D deficiency rescues axonal mitochondrial transport in Alzheimer's neurons. PLoS One 2013;8:e54914. [Crossref] [PubMed]
- Poljsak B, Suput D, Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013;2013:956792. [Crossref] [PubMed]
- Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol 2009;554:165-81. [Crossref] [PubMed]
- Wang X, Wang W, Li L, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta 2014;1842:1240-7. [Crossref] [PubMed]


