A fitting machine learning prediction model for short-term mortality following percutaneous catheterization intervention: a nationwide population-based study
Introduction
Coronary heart disease is a major public health concern, especially in developed countries (1). Consequently, percutaneous coronary intervention (PCI) is one of the most extensively and increasingly used procedures (2), and it is indicated as the primary treatment for ST-elevation myocardial infarction (STEMI) and also for non-STEMI, atypical chest pain, stable angina, unstable angina, and positive stress tests (3). With advances in technology, periprocedural complications of PCI have declined and patients often achieve favorable outcomes (4). However, mortality risk following PCI continues to be a major concern. A risk model for predicting mortality following PCI could assist clinicians in better timed and more appropriate care delivery, as well as greater awareness of high-risk groups (5,6).
Many PCI mortality risk models have been proposed (7-15). Studies have shown that incorporating angiographic features and multiple biomarkers enhances prediction power (15,16-18). Because 50% of the mortality following PCI is due to non-cardiac reasons, creating a model that accounts for general complications post PCI appears optimal for predicting in-hospital mortality (9).
In recent years, multivariate prediction models including artificial neural network models (ANN), decision trees (DT), linear discriminant analysis classifiers (LDA), logistic regression models (LR), naïve Bayes classifiers (NB), and support vector machines (SVM) have been used in medicine (19-22). Being used a nonlinear statistical model to identify variable patterns, ANNs are the most commonly used method among these methods (23-26). However, Freeman et al. used ANN to predict in-hospital deaths following percutaneous transluminal coronary angiography (27). In their study, they did not find ANN to be superior to the LR model. With the widely used and advancing techniques of percutaneous catheterization intervention, conducting a nationally representative population study to determine an appropriate prediction model for short-time mortality following PCI is of vital importance.
The Taiwan National Health Insurance Research Database (NHIRD), with >99% coverage, includes longitudinal medical and procedure information of all insured participants (28) in a database. We aimed to find the best prediction model in predicting predict 30-day risk of mortality from the pre-PCI and post-PCI data of patients with acute myocardial infarction (AMI) who underwent PCI.
Methods
Data source
Taiwan’s National Health Insurance (NHI) program began in March 1995 and now offers comprehensive medical coverage to all residents in Taiwan (29). The study cohort was selected from the Longitudinal Health Insurance Database 2000 (LHID2000) of the NHI program. The LHID2000 comprises 1,000,000 randomly sampled people enrolled in the NHI program, and it collected all medical records of these individuals from 2004 to 2013. Disease diagnoses were identified and coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM).
Ethics statement
The NHIRD encrypts each patient’s personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-CR4). The IRB also specifically waived the consent requirement.
Sampled participants
We identified patients with AMI (ICD-9-CM code 410) aged >18 years who underwent PCI intervention between January 1, 2000, and December 31, 2013. The date of the PCI intervention was assigned as the index date. A total of 3,421 patients who underwent PCI intervention including culprit lesion and multivessel PCI were enrolled in our study.
Predictive factors
Postoperative complications were defined as diagnosis or mortality occurring within 30 days after the date of PCI intervention. Complications included upper gastrointestinal bleeding, acute kidney injury that required dialysis, new onset of arrhythmia, and ICU admission. Baseline comorbidities and surgeries associated with mortality before the index date included hyperlipidemia, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, heart failure, stroke, atrial fibrillation, obesity, chronic kidney disease/end-stage renal disease, peripheral arterial occlusion disease, history of PCI, coronary artery bypass graft (CABG), and intra-aortic balloon bump (IABP). Long-term medications used at baseline were also considered, including aspirin, warfarin, and clopidogrel. The aforementioned baseline comorbidities, previous interventions, previous surgeries, and 30-day postoperative complications were confirmed on the basis of ICD-9-CM codes. Each one of these predictive factors comprise the 22 features used later in the prediction models.
Statistical analysis
Chi-square test was employed to compare the differences in the distribution of PCI type, age, sex, comorbidities, and 30-day postoperative complication between patients with and without 30-day postoperative mortality. In addition, the t-test was used to compare the differences of mean ages between the two groups.
Data construction
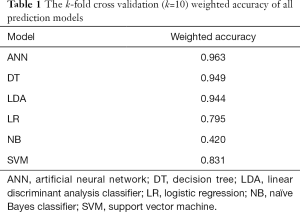
This study comprised 3,421 patients who underwent PCI, each of whom is represented by a data point. There are 22 features per data point; the features comprised of the patients’ sex, comorbidities, medications taken, and 30-day postoperative complications. To accurately determine the performance of prediction models, the patients were split into training and test sets at a ratio of approximately 9:1. Of all 3,421 patients undergoing PCI procedure, 3,079 patients were included in train set and 342 patients were included in the test set. The use of 10% of the dataset for the test set was modeled after several research papers in the field, including research done by Avati et al. (29). Instead of splitting the data into training, validation, and test subsets and performing holdout cross-validation on the validation set, we decided to split the data into training and test sets and perform k-fold cross-validation, which is an improvement over the traditional train-validation-test split cross-validation method (30). The k-fold cross validation (k=10) weighted accuracy is stated in Table 1.
Full table
The output data is unbalanced with a bias toward 30-day survival. To ensure that the prediction models did not skew toward the dominant class, weighting was applied to the data set prior to training. Since the ratio of 30-day death to 30-day survival was 1:26.368, each 30-day survival data point was weighted approximately 26 times greater than each 30-day death data point.
Algorithms and training
To select the hyperparameters that yield the best performance for each prediction model, we used k-fold cross-validation with a k value of 10. Owing to the limited data, k-fold cross-validation was used instead of holdout cross-validation.
ANN
We used a fully-connected multilayer perceptron (MLP) feedforward network to train the data. Our model consisted of one input layer of 22 dimensions, a hidden layer of 11 dimensions, and an output layer of 2 dimensions. During the model selection process, we also experimented with a different number of layers and more complicated ANNs such as convolutional neural networks and recurrent neural networks. However, we find that neither other types of neural networks nor ANNs with more layers exceed the performance of an MLP with three layers. Therefore, we chose the simplest model with the best performance.
The network was trained using stochastic gradient descent and optimized with Adam with default parameters outlined by Kingma et al. (30). Each layer used the scaled exponential linear unit activation function (31) except the output layer, which used the softmax activation function. A dropout of 20% was applied for the input layer and 50% for the output layer (32). Because the problem was a binary classification task, the categorical cross entropy error function was used as the loss function. The network was train for 1,600 epochs. The ANN model was implemented with the TensorFlow library (version 1.9.0) (33).
DT
The DT model used Gini impurity to measure the quality of split. The minimum samples per leaf was set to 1, while the minimum number of samples required to split a node was set to 2. The maximum depth was set to 7. While DTs with a larger maximum depth could be used, we found out that having a depth greater than 7 resulted in DTs that tended to overfit the train set. The DT model was implemented with the scikit-learn framework (version 0.19.1) (34).
LDA
The LDA used a singular value decomposition (SVD) solver to create a linear decision boundary. A SVD solver was used instead of an eigenvalue decomposition solver because calculating a covariance matrix with eigenvalue decomposition is slow with a large number of features (22). The LDA model was implemented with the scikit-learn framework (version 0.19.1) (34).
LR
The LR model used a L2 regularization penalty with primal formulation. Primal formulation was used because there are more samples than features. Stochastic average gradient descent was used as the optimizer. The one-vs-rest scheme was used as the loss function. The regularization strength was set to 1.0, and the model was trained for 100 iterations before convergence. The LR model was implemented using the scikit-learn library (version 0.19.1) (34) and the LIBLINEAR library (version 3.21) (35).
NB
The NB classifier is a multinomial NB. A multinomial model was used instead of a Gaussian model because all of the features, except age, are discrete. The NB software was implemented using the scikit-learn library (version 0.19.1) (34).
SVM
The SVM model is a C-support vector classification (C-SVC) model that used a radial basis function (RBF) as its kernel. We also evaluated a nu-support vector classification (nu-SVC) model, but we found a C-SVC model to have a better classification performance. In order to compare the performance of the SVM model to all other models, probability estimates were enabled to plot the ROC curve. The shrinking heuristic was enabled to save training time by bounding the optimal solution. The SVM model was implemented using the LIBSVM library (version 3.21) (36).
Evaluation of prediction model performance
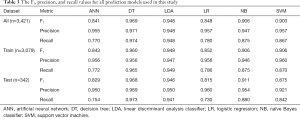
Because the data distribution was heavily skewed toward the positive class, the accuracy could not reliably measure prediction model performance (37). Instead, the weighted averaged F1, precision, and recall values were used to measure prediction model performance. These three confusion matrix metrics were calculated across all data, the train set, and the test set for each model.
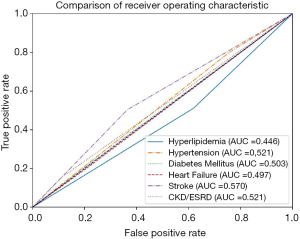
Additionally, the receiving operating characteristic (ROC) curve was also used as a metric to measure prediction model performance. The Area Under ROC (AUROC) of each model was compared with each other, and then the AUROC of the prediction models were compared with the AUROC of comorbidities with clinical relevance or association with PCI. This is done to demonstrate the necessity of using multivariate prediction models in this study. IBM SPSS 24.0 (SPSS, Inc., Chicago, IL, USA) was used to calculate the ROC and AUROC.
Results
Demographic features
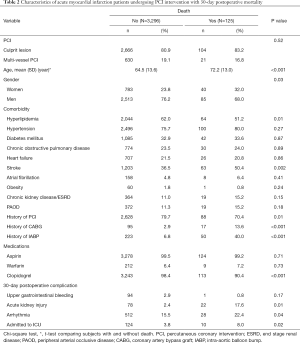
The majority of the patients underwent culprit lesion (83.2% vs. 80.9%) and were aged ≥65 years (72.0% vs. 49.2%). The mean ages of the death group and non-death groups were 72.2 and 64.5 years, respectively, and most of the patients were men. Compared with the non-death group, the death group tended to have more comorbidities, surgeries, and 30-day postoperative complications, including stroke, history of CABG and IABP, dialysis-requiring acute kidney injury, arrhythmia, and ICU admission. By contrast, compared with the death group, the non-death group was more likely to have hyperlipidemia and history of PCI, as well as to require clopidogrel medication. The characteristics of the data set are shown in Table 2.
Full table
Evaluation of prediction models
The weighted k-fold cross validation (k=10) weighted accuracy of all prediction models are listed in Table 1. The models with the highest weighted accuracies are the ANN model (0.963), DT model (0.949), and LDA model (0.944), while the model with the lowest weighted accuracy is the NB model (0.420).
The F1, precision, and recall values of all prediction models are listed in Table 3. The DT model has the highest overall F1, precision, and recall values compared to all other models. For both the LR and ANN models, the precision values are significantly higher than the recall values. Since precision represents the positive predictive value and the recall represents the negative predictive value, the LR and ANN models may be overfitting the data. This hypothesis is supported by the fact that the F1 values for the ANN and LR models are the lowest of all predictors.
Full table
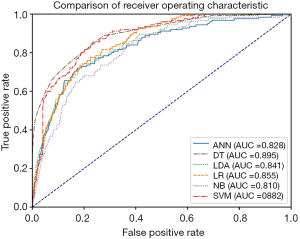
The ROC of all prediction models across all data is outlined in Table 4. The model with the highest AUROC is the DT model (0.895, 95% CI: 0.865–0.925), followed by the SVM model (0.882, 95% CI: 0.855–0.909) and the LR model (0.855, 95% CI: 0.827–0.883). The ROC curve is shown in Figure 1.
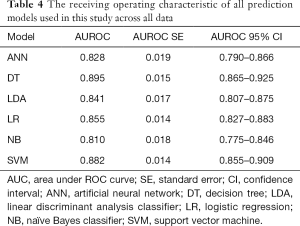
Full table
Comparison to relevant variables
The AUROC of the prediction models were compared with the AUROC of the following variables due to their clinical relevance or association to PCI: hyperlipidemia, hypertension, diabetes mellitus, heart failure, stroke, and chronic kidney disease (CKD)/ESRD. The comorbidity with the highest AUROC is stroke (0.570, 95% CI: 0.518–0.621), while the comorbidity with the lowest AUROC is hyperlipidemia (0.446, 95% CI: 0.394–0.498) (Table 5). All of the variables are close to the null hypothesis true area of 0.5. The ROC curve is shown in Figure 2. Supplementary DT showed calculating probabilities of features for patients undergoing PCI (Figure S1).
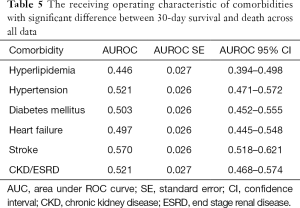
Full table
Discussion
Using the NHIRD, this pilot study demonstrated that a variety of multivariate prediction models can predict 30-day mortality following PCI, with the DT model having the most optimal performance and scalability. Although numerous studies have explored for prediction model of mortality and morbidity after PCI (16,27,38-43), two unique pilot points of our study could be addressed in enhancing the predictive accuracy of prediction model.
First, we added both pre-PCI and post-PCI comorbidity and complications as variables. In the study of Freeman et al. they found that the predictive accuracy of ANNs can be increased with variable selection but is not superior to traditional modeling (27). Their study suggests the importance of variable selection. Thus the pre-procedure variables chosen in this study were based on previous PCI-related studies (16,44-46). Further, we used diseases as proxies tightly linked with high cardiovascular risk after PCI (47-49). In the study of Ellis et al., the predictors they chosen included detailed angiographic findings and culprits vessels and baseline angiographic finding about ejection fraction of left ventricle and number of diseased (≥50% diameter stenosis) and treated vessels (46). Compared with the model of Ellis et al. (46), our model constructed based on the disease variables could be more easily assessed in this risk calculation. To increase the practical value, we decided to choose gastrointestinal bleeding, arrhythmia, and transfer to ICU care as post-procedure variables. It is interesting to note while we compare each feature, PCI patients had past stroke had highest risk of post-PCI mortality. This result would be clinically valuable in providing information for post-PCI care setting of these extremely high risk patients.
Second, we attempted to find the fitting model for prediction post-PCI mortality among current prediction models. We used confusion matrix and ROC metrics used to measure the prediction model performances in NHIRD database. We found that the model with the highest AUROC is the DT model (0.895, 95% CI: 0.865–0.925), followed by the SVM model (0.882, 95% CI: 0.855–0.909) and the LR model (0.855, 95% CI: 0.827–0.883). Therefore, we find DT model achieves a fitting prediction mode in our study. Evaluating the LR and ANN models, the precision values are significantly higher than the recall values, which means the LR and ANN models may be overfitting the data and might not be appropriate models in this study. In another aspect, we found that Multinomial naïve Bayes models allows for partial fitting of incomplete datasets. Our findings mean that NB models could be useful for a web-based classification system where datasets arrive one-by-one as opposed to all at the same time. Thus, in the NHIRD database, multinomial naïve Bayes models may be better than SVMs in terms of efficiency and scalability.
Ellis et al. used LR to calculate the operator-specific mortality prediction in patients following PCI (46). The area under curve of mortality is 0.85, which is comparable to our results (46). Our LR model improves upon their methodology by enabling dynamic prediction since we have incorporated the post-procedure variables. While LDA classification models are relatively uncommon in the medical field, DT models have been widely implemented in applications where a white-box classification is necessary (50). The decision path of any given data point can be determined for DTs, which makes it suitable for applications where such a decision path is necessary, such as in clinical pathology. LR models are also suitable for such applications since one can also draw statistical inferences from prediction model outputs (19).
This study had several advantages. First, because DT models are advantageous in dynamical prediction based on variable selection, we included both baseline demographics and in-hospital newly occurred complications of each patient. Thus, our model can be employed for predicting pre- and post-PCI mortality risk during hospitalization. This model could assist clinicians in their awareness of potential mortality risks when new events occur among post-PCI patients staying in hospital. Second, the NHIRD is a national database in Taiwan; therefore, the possibility of selection bias such as in a single-center database was alleviated when constructing the PCI mortality model. Third, we selected patients with AMI as our study group. Peterson et al. found that PCI in-hospital mortality was 1.27%, ranging from 0.65% in elective PCI to 4.81% in STEMI patients (16). Because mortality following PCI differed among patients with different PCI indications, patients with AMI were selected as the study cohort to effectively fulfill the critical need for interventional cardiology.
Several limitations must be mentioned. First, information regarding body mass index, HbA1C, levels of high-density and low-density lipoproteins, dietary preference, exercise, family history of heart diseases, smoking, electrocardiogram, blood pressure upon AMI, left ventricular end-diastolic pressure, ejection fraction, and culprit vessels were unavailable through the NHIRD. Furthermore, information about angiographic features and biomarkers of each patient is unobtainable in NHIRD, thus we could not corporate these data as the study features. Those missing information might have impacts on formation of prediction models. However, although we did not have such detailed information, our model achieved an AUC of 0.895 and a precision of 0.971 for the DT model on the basis of diagnostic and procedure codes. Our study demonstrated the advantages of simplicity and flexibility in assessing the mortality risk of patients with PCI. Second, although we used a validation set, it was extracted from the same database. External validation with larger data sets encompassing 20-year durations of the NHIRD are required to validate our findings. It would be useful to use the observations from 2004 to 2008 and do testing by using the observations from 2009 to 2013. However, the number of patients would be not large enough in each divided set. Third, the majority of those insured by the NHI program are Taiwanese; thus, our model which is constructed on single-country data might not be generalizable to other countries. Finally, using post-PCI complications as predictors might limit the usability of the algorithm to post-PCI settings, which should also be mentioned here.
Conclusions
This study reported that DT models can be applied to the NHIRD in order to predict 30-day mortality following PCI in patients with AMI. We hope work could provide insights for further studies about applying prediction model on ICD-coded database. This model may enable more dynamic and timely predictions of mortality during hospitalization. Additional studies are necessary for external validation and to test the applicability of this model in patients undergoing PCI without AMI.
Acknowledgments
Funding: This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital (DMR-107-192), Academia Sinica Stroke Biosignature Project (BM10701010021), MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005), Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The investigation conforms to the principles outlined in the Declaration of Helsinki. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115-CR4). The IRB also specifically waived the consent requirement.
References
- Sanchis-Gomar F, Perez-Quilis C, Leischik R, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256. [Crossref] [PubMed]
- Cram P, House JA, Messenger J, et al. Indications for percutaneous coronary interventions performed in US hospitals: a report from the NCDR®. Am Heart J 2012;163:214-21.e1. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. [Crossref] [PubMed]
- Applegate RJ, Sacrinty MT, Kutcher MA, et al. Trends in vascular complications after diagnostic cardiac catheterization and percutaneous coronary intervention via the femoral artery, 1998 to 2007. JACC Cardiovasc Interv 2008;1:317-26. [Crossref] [PubMed]
- Spoon DB, Lennon RJ, Psaltis PJ, et al. Prediction of cardiac and noncardiac mortality after percutaneous coronary intervention. Circ Cardiovasc Interv 2015;8:e002121. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082-115. [Crossref] [PubMed]
- Brennan JM, Curtis JP, Dai D, et al. Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv 2013;6:790-9. [Crossref] [PubMed]
- Gevaert SA, De Bacquer D, Evrard P, et al. Gender, TIMI risk score and in-hospital mortality in STEMI patients undergoing primary PCI: results from the Belgian STEMI registry. EuroIntervention 2014;9:1095-101. [Crossref] [PubMed]
- Ellis SG, Shishehbor MH, Kapadia SR, et al. Enhanced prediction of mortality after percutaneous coronary intervention by consideration of general and neurological indicators. JACC Cardiovasc Interv 2011;4:442-8. [Crossref] [PubMed]
- Tanaka S, Sakata R, Marui A, et al. Predicting long-term mortality after first coronary revascularization: – the Kyoto model –. Circ J 2012;76:328-34. [Crossref] [PubMed]
- Resnic FS, Normand SL, Piemonte TC, et al. Improvement in mortality risk prediction after percutaneous coronary intervention through the addition of a "compassionate use" variable to the National Cardiovascular Data Registry CathPCI dataset: a study from the Massachusetts Angioplasty Registry. J Am Coll Cardiol 2011;57:904-11. [Crossref] [PubMed]
- Hamburger JN, Walsh SJ, Khurana R, et al. Percutaneous coronary intervention and 30-day mortality: the British Columbia PCI risk score. Catheter Cardiovasc Interv 2009;74:377-85. [Crossref] [PubMed]
- Dégano IR, Subirana I, Torre M, et al. A European benchmarking system to evaluate in-hospital mortality rates in acute coronary syndrome: the EURHOBOP project. Int J Cardiol 2015;182:509-16. [Crossref] [PubMed]
- Yu J, Mehran R, Clayton T, et al. Prediction of 1-year mortality and impact of bivalirudin therapy according to level of baseline risk: A patient-level pooled analysis from three randomized trials. Catheter Cardiovasc Interv 2016;87:391-400. [Crossref] [PubMed]
- Iqbal J, Vergouwe Y, Bourantas CV, et al. Predicting 3-year mortality after percutaneous coronary intervention: updated logistic clinical SYNTAX score based on patient-level data from 7 contemporary stent trials. JACC Cardiovasc Interv 2014;7:464-70. [Crossref] [PubMed]
- Peterson ED, Dai D, DeLong ER, et al. Contemporary Mortality Risk Prediction for Percutaneous Coronary Intervention: Results from 588,398 Procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol 2010;55:1923-32. [Crossref] [PubMed]
- Damman P, Kampinga MA, van der Horst IC, et al. Multiple biomarkers for the prediction of short and long-term mortality after ST-segment elevation myocardial infarction: the Amsterdam Groningen collaboration. J Thromb Thrombolysis 2013;36:42-6. [Crossref] [PubMed]
- Sinning JM, Asdonk T, Erlhofer C, et al. Combination of angiographic and clinical characteristics for the prediction of clinical outcomes in elderly patients undergoing multivessel PCI. Clin Res Cardiol 2013;102:865-73. [Crossref] [PubMed]
- Ayer T, Chhatwal J, Alagoz O, et al. Comparison of logistic regression and artificial neural network models in breast cancer risk estimation. Radiographics 2010;30:13-22. [Crossref] [PubMed]
- Šter B, Dobnikar A. editors. Neural networks in medical diagnosis: Comparison with other methods. International Conference on Engineering Applications of Neural Networks, 1996.
- Soni J, Ansari U, Sharma D, et al. Predictive data mining for medical diagnosis: An overview of heart disease prediction. Int J Comput Appl 2011;17:43-8.
- Polat K, Güneş S. Breast cancer diagnosis using least square support vector machine. Digital Signal Processing 2007;17:694-701. [Crossref]
- Tu JV. Advantages and disadvantages of using artificial neural networks versus logistic regression for predicting medical outcomes. J Clin Epidemiol 1996;49:1225-31. [Crossref] [PubMed]
- Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform 2002;35:352-9. [Crossref] [PubMed]
- Amato F, López A, Peña-Méndez EM, et al. Artificial neural networks in medical diagnosis. J Appl Biomed 2013;11:47-58. [Crossref]
- Kickingereder P, Isensee F, Tursunova I, et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol 2019;20:728-40. [Crossref] [PubMed]
- Freeman RV, Eagle KA, Bates ER, et al. Comparison of artificial neural networks with logistic regression in prediction of in-hospital death after percutaneous transluminal coronary angioplasty. Am Heart J 2000;140:511-20. [Crossref] [PubMed]
- Bureau of National Health Insurance--Management Measures for Out-of-Pocket Payments by NHI. Available online: . accessed on December 5, 2019.https://www.nhi.gov.tw/english/News_Content.aspx?n=996D1B4B5DC48343&sms=F0EAFEB716DE7FFA&s=4FF5D8FCEC99BA54
- Avati A, Jung K, Harman S, et al. Improving palliative care with deep learning. BMC Med Inform Decis Mak 2018;18:122. [Crossref] [PubMed]
- JSFFE. Available online: https://www.cs.cmu.edu/~schneide/tut5/node42.html
- Klambauer G, Unterthiner T, Mayr A, et al. Available online: https://papers.nips.cc/paper/6698-self-normalizing-neural-networks
- Srivastava N, Hinton G, Krizhevsky A, et al. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 2014;15:1929-58.
- Abadi M, Barham P, Chen J, et al. editors. TensorFlow: A System for Large-Scale Machine Learning. OSDI, 2016.
- Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: Machine learning in Python. J Mach Learn Res 2011;12:2825-30.
- Fan RE, Chang KW, Hsieh CJ, et al. LIBLINEAR: A library for large linear classification. J Mach Learn Res 2008;9:1871-4.
- Chang CC, Lin CJ. LIBSVM: a library for support vector machines. ACM transactions on intelligent systems and technology (TIST), 2011;2:27.
- He H, Garcia EA. Learning from imbalanced data. IEEE Trans Knowl Data Eng 2009;21:1263-84. [PubMed]
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol 2004;44:1393-9. [PubMed]
- Halkin A, Singh M, Nikolsky E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. J Am Coll Cardiol 2005;45:1397-405. [Crossref] [PubMed]
- Duffy BK, Gurm HS, Rajagopal V, et al. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol 2006;97:993-6. [Crossref] [PubMed]
- Laskey WK, Jenkins C, Selzer F, et al. Volume-to-creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol 2007;50:584-90. [Crossref] [PubMed]
- Valgimigli M, Serruys PW, Tsuchida K, et al. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol 2007;99:1072-81. [Crossref] [PubMed]
- Addala S, Grines CL, Dixon SR, et al. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score). Am J Cardiol 2004;93:629-32. [Crossref] [PubMed]
- Hannan EL, Arani DT, Johnson LW, et al. Percutaneous transluminal coronary angioplasty in New York State. Risk factors and outcomes. JAMA 1992;268:3092-7. [Crossref] [PubMed]
- Kimmel SE, Berlin JA, Strom BL, et al. Development and validation of simplified predictive index for major complications in contemporary percutaneous transluminal coronary angioplasty practice. The Registry Committee of the Society for Cardiac Angiography and Interventions. J Am Coll Cardiol 1995;26:931-8. [Crossref] [PubMed]
- Ellis SG, Omoigui N, Bittl JA, et al. Analysis and Comparison of Operator-Specific Outcomes in Interventional Cardiology. Circulation 1996;93:431. [Crossref] [PubMed]
- Influence of diabetes on 5-year mortality and morbidity in a randomized trial comparing CABG and PTCA in patients with multivessel disease: the Bypass Angioplasty Revascularization Investigation (BARI). Circulation 1997;96:1761-9. [Crossref] [PubMed]
- Tompkins C, Mclean R, Cheng A, et al. End‐stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011;22:1099-104. [Crossref] [PubMed]
- Breuer AC, Furlan AJ, Hanson MR, et al. Central nervous system complications of coronary artery bypass graft surgery: prospective analysis of 421 patients. Stroke 1983;14:682-7. [Crossref] [PubMed]
- Anyanwu MN, Shiva SG. Comparative analysis of serial decision tree classification algorithms. International Journal of Computer Science and Security 2009;3:230-40.