The pathogenic AGT c.856+1G>T mutation of a patient with multiple renal cysts and hypertension
Introduction
Angiotensinogen (AGT) is the precursor of angiotensin peptides in the renin-angiotensin system (RAS) (1). The RAS plays a key role in regulating blood pressure and physiological function of the kidney. The human AGT gene is located on chromosome 1q42 and composed of 5 exons (4 coding exons) (1). Several mutations of AGT gene (rs74315283; rs121912702; rs387906578) have been reported to cause a severe fetal disorder autosomal recessive renal tubular dysgenesis (RTD; OMIM 267430) (2,3). Also, the AGT gene was associated with essential hypertension (EHT; OMIM 145500) in many studies.
EHT is the most common type of hypertension, accounting for almost 95% of all hypertensive cases. EHT is a heterogeneous disease of which the exact pathogenesis is still unknown. At present, people think this disorder is caused by interactions between the environment and genetic factors (4). In the past few years, with the development of genetic testing technologies, people have paid more attention to genetic factors of hypertension. The genetic linkage between the AGT gene and EHT has been widely studied in the past few decades (5). Case-control studies and systematic reviews from different races have shown that several variants of the AGT gene are more common in EHT patients (6,7).
Cystic kidney disease is another heterogeneous disease characterized by the formation of fluid-filled sacs in the kidneys (8). This disorder is a key cause of chronic end-stage renal disease (ESRD) (9). Among this complex disorder, several types have been confirmed to be hereditary cystic kidney diseases, such as autosomal recessive polycystic kidney disease (ARPKD), nephronophthisis, and Bardet-Biedl syndrome (10). Abnormal expression of angiotensinogen was found to be associated with the formation of cysts in ARPKD mouse model (11).
Tabei et al. conducted a case-control study and found that the allele frequency of AGT-TT genotype (M235T) differed significantly from patients with both hypertension and simple renal cysts and normal individuals (12). This means the AGT gene may be involved in both hypertension and renal cyst formation. Here, we present a case of an intron mutation of the AGT gene in a patient with both hypertension and multiple renal cysts.
Case presentation
Clinical history and laboratory data
The patient was a 29-year-old male Chinese, and a mildly elevated serum creatinine level was found on laboratory evaluation beginning in 2010 because of his lower back pain caused by urolithiasis. One week later, the patient's serum creatinine returned to 130 µmol/L (normal range 44–133 µmol/L) and urine protein was negative in the local hospital. Ultrasonography displayed multiple cystic structures of both kidneys, a hamartoma, and several calculi in the right kidney. His serum creatinine remained at 130–160 µmol/L over the next few years, and urine protein levels were maintained between “trace” and “1+”. Also, elevated blood pressure was found in his first hospitalization; however, detailed blood pressure level data were unavailable.
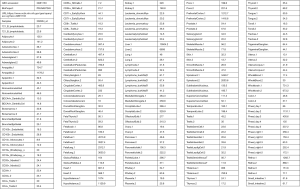
In 2014, the patient’s other ultrasonography showed a hypoechoic nodule and multiple echogenic nodules in his right kidney, and multiple cystic structures in both kidneys. Two months later, in 2015, he went to another hospital for aggravated lower abdominal distension. An enhanced helical CT scan of the kidneys showed a clearer image of multiple kidney cysts: this patient had 11 cysts in the right kidney and 5 cysts in the left kidney (Figure 1A). He then accepted laparoscopic partial nephrectomy of the right kidney. The diagnostic report on pathologic tissues at urology surgery in 2015 indicated renal angioleiomyoma of the right kidney (Figure S1). In this hospitalization, his serum creatinine levels swung between 140 and 165 µmol/L, urine protein was detected as “1+” several times, and the highest systolic blood pressure was 150 mmHg.
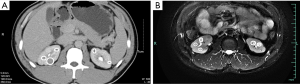
He was later referred to our nephrology outpatient clinic in 2017. Detailed examination showed a serum creatinine level of 166 µmol/L (normal range 59–104 µmol/L), estimated glomerular filtration rate (eGFR) of 47.27 ml/min using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) [based on serum creatinine] equation, and urine protein 0.7 g/L (normal range 0.010–0.140 g/L) (Table 1). Routine blood tests showed an erythrocytosis, and bone marrow cytology displayed active erythroid hyperplasia (Table S1). We tested for the JAK2 mutation (p.V617F) in the patient but found no such mutation. The result of magnetic resonance imaging (MRI) in kidneys showed that this patient had 8 cysts in the right kidney (postoperative) and 7 cysts in the left kidney (Figure 1B). On ultrasound, the kidneys were echogenic and borderline small, measuring 9.9 cm on the right and 8.8 cm on the left, and there were multiple cystic structures of various sizes in both kidneys, with the biggest being nearly 1.2 cm × 1.5 cm in the right kidney. Multiple small stones were present in both kidneys, and multiple echogenic nodules were in the right kidney (hamartoma); however, no cysts were found in the liver (Figure 2). There were no abnormalities in his parents’ kidneys or liver ultrasonography (Figure S2). Physical examination showed a blood pressure of 144/110 mm Hg. His mother was diagnosed with hypertension lasting for over 20 years.

Full table

Full table
The patient’s medical history included gout and renal hamartoma. Medications included allopurinol tablets 0.2 g and sodium bicarbonate 0.5 g daily for 2 years. Due to the ultrasonography changes in this patient’s kidneys and continuous renal function impairment with hypertension, to identify simple renal cyst and polycystic kidney disease, we performed a gene test. The sequencing analysis of the patient and his parents revealed a heterozygous intron mutation in the AGT gene (c.856+1G>T), and no variants in PKD1, PKD2, or PKHD1 were detected. PKD1 gene has 7 pseudogenes that make its coverage in exome sequencing insufficient to exclude the presence of pathogenic variation. Thus, further experimentation applying long-PCR and Sanger sequencing to screen PKD1 variations is underway. Apart from this, this patient had both renal hamartoma and renal cysts, so we needed to distinguish it from tuberous sclerosis. However, no variants in TSC genes were detected. We also performed immunohistochemical staining of AGT protein using paraffin sections of surgically removed tissue in 2015, but we could not detect kidney tissues under the microscope (data not shown). To verify whether this mutation can affect angiotensinogen protein expression, we used an enzyme-linked immunosorbent assay (ELISA) to measure the serum levels of angiotensinogen. Results showed that the patient and his mother had lower serum angiotensinogen levels than his father (Table 1). We tested the RAS system of the patient and his parents (Table 2). We found that the patient and his mother had significantly higher levels of angiotensin I-converting enzyme (ACE) and aldosterone than his father. The patient, in particular, had a significantly higher level of ACE than normal.

Full table
Diagnosis and treatments
Chronic kidney disease stage III, cystic kidney disease, and essential hypertension.
Clinical follow-up
Following the diagnosis, the patient accepted medications of losartan potassium 100 mg/corbrin 0.4 g daily for one week. At the time of patient discharge, the creatinine level improved slightly to 165 µmol/L (estimated GFR, 47.62 mL/min), blood pressure was 110/77 mmHg, and proteinuria decreased to 0.2 g/L. At his latest evaluation, 6 months after discharge, serum creatinine level was 185 µmol/L (estimated GFR, 41.17 mL/min) with proteinuria at 1.0 g/L and blood pressure at 123/83 mmHg.
Mutation analysis of the AGT gene
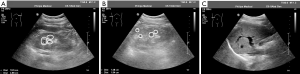
The sequence analysis of the patient and his parents revealed a heterozygous intron mutation in the AGT gene (c.856+1G>T) (Supplemental File). The mutation was localized in an evolutionary conservation nucleotide (IVS856+1) of intron 2 (Figure 3A,B,C). Sequence analysis showed that the variant was inherited from his mother. Next, we performed Sanger sequencing in the proband and his parents for the validation of the AGT gene variant (Figure 3D). Forward AGT primer: CTGTGGATGAAAAGGCCCTA, reverse AGT primer: ACCCCAGTTCCTGACCTTCT.
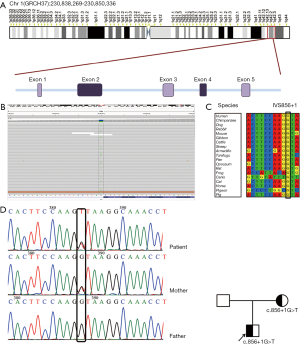
Functional changes prediction of c.856+1G>T mutation
This splice variant destroys the established splice donor site, and the ClinVar database predicted this mutation might lead to an abnormal message or an abnormal protein product. Web-based software MutationTaster predicted this mutation was disease-causing, capable of causing splice site change and affecting protein features (Figure 4). Human splicing finder 3.1 (HSF 3.1) is a software to identify and predict mutations’ effect on splicing motifs. The results of its algorithms indicated that the c.856+1G>T mutation may break a normal donor splice site and create a new donor splice site (Figure 5). Also, SPIDEX index predicted the mutation could probably change splicing, while Combined Annotation Dependent Depletion (CADD) score predicted the mutation might be a pathogenic one (Table 3). Since mRNA of AGT gene was faintly expressed in peripheral blood and blood cells as demonstrated in another study (Figure S3) (13), we did not make a polymerase chain reaction to verify whether the mRNA was affected. The c.856+1G>T variant is not observed at a significant frequency in large population cohorts (14,15).
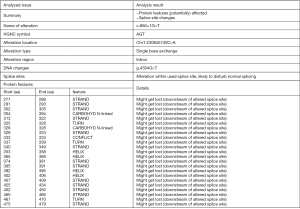


Full table
Discussion
AGT is the precursor of angiotensin peptides in the renin-angiotensin system (RAS), and plays a key role in regulating blood pressure and renal physiological function as an essential member of the RAS (1). The human AGT gene is located on chromosome 1q42 and is composed of 5 exons, and several pathogenic variants of the AGT gene have been observed. Among these deleterious allelic variants are those associated with susceptibility to essential hypertension (NM_000029.3: p.T242I, p.L244R, and p.Y281C) (6,7). With the popularization of gene test application in clinical diagnosis and therapy, more AGT gene single-nucleotide polymorphisms have been detected, but most of them are non-pathogenic (16).
In this report, we found a previously reported intron mutation site of the AGT gene in a 29-year-old male Chinese with multiple renal cysts and hypertension. It is a heterozygous mutation inherited from the mother. The clinical symptoms of this patient were special; hypertension and renal impairment were found at an early age but progressed slowly. His creatinine level has remained at 130–160 µmol/L for many years, urine protein levels have been maintained between “trace” and “1+,” and medical imaging examination has displayed multiple cystic structures in both kidneys. The DNA sequencing analysis of the patient showed no mutation in polycystic kidney disease or tuberous sclerosis relative genes, so he was diagnosed with cystic kidney disease.
Additionally, routine blood tests and bone marrow cytology suggested erythrocytosis in this patient, and no V617F mutation in the JAK2 gene was detected, we excluded polycythemia vera and considered it a secondary erythrocytosis resulting from focal renal ischemia caused by renal cyst compression (17). Because of his slowly progressing renal impairment and the fact that there was no manifestation of hematuria, we considered that neither renal cell carcinoma nor hamartoma could cause these clinical symptoms. It is believed that the RAS is activated in polycystic kidney disease, and perhaps due to renal ischemia caused by cyst expansion (18). Tabei et al. completed a case-control study and found that the allele frequency of AGT-TT genotype (M235T) differed significantly between patients with both hypertension and simple renal cysts and normal individuals (12). Animal experiments using Pkd1 and Pkd2 mice indicated that blockade angiotensinogen could attenuate cystogenesis (19,20). In summary, we thought the renal cysts might be the cause of renal function injury in this patient, and cystogenesis had a relationship with the c.856+1G>T mutation. Future studies are needed to elucidate the mechanisms through which this association is mediated.
The patient developed hypertension at an early age (nearly 20 years old), and his mother, carrying the same heterozygous mutation of AGT gene, also developed hypertension at a relatively early age (nearly 30 years old) compared with other hypertensive patients. We therefore speculated the c.856+1G>T mutation in AGT gene was also involved in their status of hypertension. On the other hand, we thought that the “two-hit” model proposed for cystogenesis in autosomal dominant polycystic kidney disease (ADPKD) could also explain the intra-familial phenotypic variability of multiple cystic structures being found on ultrasound in the patient’s kidneys, but no abnormalities being found in his mother’s kidneys (19,20). This hypothesis speculates that the cystogenesis in ADPKD includes two processes: the “first hit” is the germline mutation of an ADPKD gene, and the “second hit” refers to the somatic gene mutation affected by acquired factors such as infection. Here, the proband and his mother carried the same heterozygous mutation site of AGT gene; however, no cysts were detected in the mother’s kidneys, which may because she has not yet experienced the “second hit” stage.
To confirm the effect of this intron mutation on angiotensinogen protein expression, we tried to detect the cDNA fragments of different lengths of the AGT gene by PCR amplification and agarose gel electrophoresis using RNA isolated from peripheral blood cells; however, we failed to amplify the target AGT gene due to its low expression pattern in blood cells. Immunohistochemical staining of AGT protein was also performed using paraffin sections of surgically removed tissue of the patient in 2015. Regrettably, no kidney tissues were found under the microscope. ELISA measurement showed that the patient had a lower serum angiotensinogen level than his father (1,467.23 vs. 2,452.64 pg/mL), and that the serum angiotensinogen concentration of his mother was also lower than the father (1,542.73 vs. 2,452.64 pg/mL). We cannot conclude that the mutation is associated with plasma angiotensinogen level since the research sample size is too small. By testing the RAS system of the patient and his parents with the normal levels of plasma renin, we found that the patient and his mother had significantly higher levels of ACE and aldosterone than his father; the patient, in particular, had a significantly higher level of ACE than normal.
Interestingly, with the further use of online software to predict the potential functional changes of this mutation site, various algorithms all indicated that the c.856+1G>T mutation in the AGT gene has the possibility of destroying the canonical splice donor site of intron 2 and affecting the features of the protein. MutationTaster, Human Splicing Finder 3.1, and SPIDEX made the same prediction that this mutation site may either leading to an abnormal message that is subject to nonsense-mediated mRNA decay, or to an abnormal protein product if the message is used for protein translation. After the literature search, we found this variant was not observed at a significant frequency in large population cohorts and has not been reported previously as a pathogenic variant nor as a benign variant.
In conclusion, we present a case of an intron mutation of the AGT gene in a 29-year-old male Chinese patient with multiple renal cysts and hypertension; it is a heterozygous mutation inherited from his mother. The patient’s renal function impairment progressed slowly. His renal cystic lesion and hypertension seems to be due to the presence of a heterozygous c.856+1G>T mutation in the AGT gene. However, no direct experimental evidence proved that this mutation will affect angiotensinogen protein structure or function. Furthermore, we would like to confirm this notion in an animal model for hypertension in addition to performing a c.856+1G>T mutation of the AGT gene to see whether it affects renal cyst formation in a vitro cyst model.
Supplementary
Materials and methods
Editorial policies and ethical considerations
All procedures performed in this study involving human participants were in accordance with the ethical standards of the research ethics committee of the First Affiliated Hospital of Zhejiang University and with the Helsinki declaration. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
DNA isolation and DNA quantification and qualification
Genomic DNA was extracted from 3 ml peripheral blood of the patient and his parents using Lab-Aid® 824 DNA Extraction Kit (Zeesan Biotech, Xiamen, China).
DNA purity was checked using the Nanodrop 2000® spectrophotometer (ThermoScientific, CA, USA). The DNA concentration was measured using the Qubit dsDNA HS Assay Kit on the Qubit® 3.0 Fluorometer (Life Technologies, CA, USA).
Library preparation for sequencing
A total amount of 1–3 µg DNA per sample was used as input material for the DNA sample preparations. Firstly, fragmentation was carried out using 1X Low TE Buffer (Life Technologies, CA, USA) in Covaris M220® ultrasonicator (Covaris, MA, USA) to obtain 150–200 bp DNA fragments. Subsequently, DNA fragments were purified with AMPure XP® system (Beckman Coulter, Beverly, MA, USA). Remaining overhangs were converted into blunt ends via DNA polymerase activities. After adenylation of 3’ ends of DNA fragments, SureSelect Adaptor Oligo Mix (Agilent, CA, USA) were ligated to prepare for hybridization. PCR was then performed with HercµlaSE II Fusion DNA Polymerase (Agilent, CA, USA), SureSelect Primer (Agilent, CA, USA), and SureSelect Reverse Prime (Agilent, CA, USA). Products were then purified with AMPure XP system, and concentration was measured on the Qubit® 3.0 fluorometer. At last, the library hybridization was performed in the SureSelect Hyb buffer system and captured in the SeueSelect Library system; target enrichment and amplification, and barcode labeling were done following the manufacturer’s recommendations.
Sequencing and analysis
The libraries were sequenced on an Illumina Hiseq 2500 (Illumina, San Diego, CA, USA) platform, and 75 bp paired-end reads were generated. Data analysis was made according to the Joingenome-ExomeSeq-V.1.1 (Joingenome Diagnosis, Hangzhou, China) analysis process. The cleaned reads were mapped to the human genome hg19 by BWA 0.7.12-R1039. SNPs were called out using GenomeAnalysis TK-3.3-0 and annotated with several public databases such as dbSNP (snp147), 1000 Genome (2015aug), ClinVar, ExAC, and OMIM. Online software including MutationTaster, CADD, and HSF 3.1 were used to predict the pathogenicity of the mutation.
ELISA analysis
To prepare samples for ELISA, 3 mL of whole blood per sample in VACUETTE® Z Serum Sep Clot Activator (Greiner Bio-One International, Frickenhausen, Germany) tubes were clotted for 1 h, and then centrifuged for 10 min at 1,000 g. Aspirate supernatant was aliquoted into small tubes and stored at −80 °C until use. Human Serpin A8/AGT DuoSet ELISA kit (R&D Systems, Minneapolis, MN, USA) was used to detect the serum AGT concentration. ELISA assays were performed according to protocols provided by the kit manufacturer.
Immunohistochemical analysis
Immunohistochemical analysis was performed on paraffin sections of surgically removed tissue of the patient in 2015; donor kidney tissue was used as normal control. To summarize, paraffin-embedded tissue slides were deparaffinized, rehydrated, and subjected to antigen unmasking by sodium citrate (pH 6.0) for 10 min at a sub-boiling temperature. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide for 10 min. Sections were blocked with 5% BSA for 30 min at room temperature, followed by incubation with primary antibody overnight at 4 °C. For negative control, the anti-AGT antibody was replaced with 5% BSA. Sections were incubated with a horseradish peroxidase-conjugated secondary antibody for 30 min at room temperature. At last, sections were stained with the 3’-diaminobenzidine substrate and counterstained with hematoxylin, and dehydrated. Anti-AGT antibody was from NovusBio (cat: NBP1-30027SS, CA, USA).
Acknowledgments
We are grateful to all the staff at the Kidney Disease Center of the First Affiliated Hospital of Zhejiang University for their sincere support.
Funding: This work was supported by the National Natural Science Foundation of China (81770752 to J Chen, 81470938, 81770697 to H Jiang), and the Zhejiang Provincial Natural Science Foundation of China (LQ19H050004 to W Chen).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The study was approved by the Ethical Committee of the Zhejiang University College of Medicine, the First Affiliated Hospital. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Fukamizu A, Takahashi S, Seo MS, et al. Structure and expression of the human angiotensinogen gene. Identification of a unique and highly active promoter. J Biol Chem 1990;265:7576-82. [PubMed]
- Gribouval O, Gonzales M, Neuhaus T, et al. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 2005;37:964-8. [Crossref] [PubMed]
- Uematsu M, Sakamoto O, Nishio T, et al. A case surviving for over a year of renal tubular dysgenesis with compound heterozygous angiotensinogen gene mutations. Am J Med Genet A 2006;140:2355-60. [Crossref] [PubMed]
- Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 2000;101:329-35. [Crossref] [PubMed]
- Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell 1992;71:169-80. [Crossref] [PubMed]
- Li H, Du Z, Zhang L, et al. The relationship between angiotensinogen gene polymorphisms and essential hypertension in a Northern Han Chinese population. Angiology 2014;65:614-9. [Crossref] [PubMed]
- Reiter LM, Christensen DL, Gjesing AP. Renin angiotensinogen system gene polymorphisms and essential hypertension among people of West African descent: a systematic review. J Hum Hypertens 2016;30:467-78. [Crossref] [PubMed]
- Cramer MT, Guay-Woodford LM. Cystic kidney disease: a primer. Adv Chronic Kidney Dis 2015;22:297-305. [Crossref] [PubMed]
- Chijioke A, Aderibigbe A, Olarenwaju TO, et al. Prevalence and pattern of cystic kidney diseases in Ilorin, Nigeria. Saudi J Kidney Dis Transpl 2010;21:1172-8. [PubMed]
- König JC, Titieni A, Konrad M, et al. Network for Early Onset Cystic Kidney Diseases-A Comprehensive Multidisciplinary Approach to Hereditary Cystic Kidney Diseases in Childhood. Front Pediatr 2018;6:24. [Crossref] [PubMed]
- Saigusa T, Dang Y, Mullick AE, et al. Suppressing angiotensinogen synthesis attenuates kidney cyst formation in a Pkd1 mouse model. FASEB J 2016;30:370-79. [Crossref] [PubMed]
- Tabei SM, Nariman A, Daliri K, et al. Simple renal cysts and hypertension are associated with angiotensinogen (AGT) gene variant in Shiraz population (Iran). J Renin Angiotensin Aldosterone Syst 2015;16:409-14. [Crossref] [PubMed]
- Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 2004;101:6062-67. [Crossref] [PubMed]
- Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285-91. [Crossref] [PubMed]
- 1000 Genomes Project Consortium, Auton A, Brooks LD, et al. A global reference for human genetic variation. Nature 2015;526:68-74.
- Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res 2015;43:D789-98. [Crossref] [PubMed]
- Stein BL, Oh ST, Berenzon D, et al. Polycythemia Vera: An Appraisal of the Biology and Management 10 Years After the Discovery of JAK2 V617F. J Clin Oncol 2015;33:3953-60. [Crossref] [PubMed]
- Chapman AB, Johnson A, Gabow PA, et al. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med 1990;323:1091-6. [Crossref] [PubMed]
- Fitzgibbon WR, Dang Y, Bunni MA, et al. Attenuation of accelerated renal cystogenesis in Pkd1 mice by renin-angiotensin system blockade. Am J Physiol Renal Physiol 2018;314:F210-8. [Crossref] [PubMed]
- Ravichandran K, Ozkok A, Wang Q, et al. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am J Physiol Renal Physiol 2015;308:F349-57. [Crossref] [PubMed]