Diagnostic accuracy of specific IgG antibodies for bird fancier’s lung: a systematic review and meta-analysis
Introduction
Bird fancier’s lung (BFL), a type of hypersensitivity pneumonitis (HP), is a complex syndrome caused by lung inflammation in response to avian proteins inhaled from avian droppings, serum, and feathers (1). The prevalence of BFL is reported to be 20 to 20,000 per 100,000 at-risk persons (2). Further, the incidence of HP mortality, including that from BFL, has increased recently (3). The diagnosis of BFL is made in patients with a history of avian exposure using a combination of serologic assays, radiological and pathological findings, and an antigen-avoidance trial (4).
In particular, serological assays target specific anti-avian immunoglobulin G (IgG) antibodies using various methods, such as the Ouchterlony double-immunodiffusion, enzyme-linked immunosorbent assay (ELISA), electrosyneresis, and ImmunoCAP methods (5). Although IgG-specific serologic assays are considered critical for accurately diagnosing BFL, their diagnostic accuracies remain controversial (6-9). Indeed, a recent survey demonstrated that 40% of healthy bird breeders tested positive for anti-avian antibodies using available serologic assays, which raises questions about the validity of such tests for diagnostic purposes (10). In this systematic review, the diagnostic value of specific IgG serologic assays in the diagnosis of BFL was assessed.
Methods
Data sources and searches
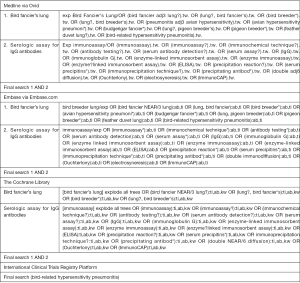
The following electronic bibliographic databases were searched: Medline, Embase, the Cochrane Library, and the International Clinical Trials Registry Platform. The search strategy included terms relating to or describing BFL (i.e., BFL, bird breeder’s lung, avian HP, feather duvet lung, bird-related HP, budgerigar fancier’s lung, and pigeon breeder’s lung) and serologic assays for IgG antibodies (precipitation reaction, Ouchterlony method, electrosyneresis method, ELISA, and ImmunoCAP assay; Figure S1). There were no restrictions concerning language, the publication year, or the type of publication. In addition, the references of the included publications were searched, and the Web of Science citation search function was used to identify additional articles. This systematic review was completed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Table S1). The protocol was preregistered in the International Prospective Register of Systematic Reviews (CRD42019122441). We used data from previous published articles in which the investigators state they obtained informed consent from the patients. Thus, no ethical approval was needed for our systematic review.

Full table
Study selection
Firstly, the title and abstracts of potential articles were independently screened by two pulmonologists, A Shiroshita and Y Tanaka, using the inclusion criteria. Secondly, the full manuscripts of the included articles were obtained and assessed by the same two independent reviewers, irrespective of whether the obtained articles were included. The inclusion criteria were as follows: prospective or retrospective cohort studies, or case-control studies assessing the sensitivity and specificity of serologic assays for anti-avian IgG antibodies (Ouchterlony method, ELISA, electrosyneresis method, and ImmunoCAP assay) among patients with suspected BFL. If there was a disagreement between the two reviewers with regard to a publication’s inclusion, it was solved by discussion or consultation with another pulmonologist (K Nakashima).
Data extraction and study quality
The following data were extracted: author, publication type, publication year, countries where the studies were performed, information on study participants, analytical methods used with the antibodies, BFL subtype (acute or chronic), setting where the studies were performed (private or university hospital), BFL diagnostic criteria, and antibody test results (true positive, true negative, false positive, and false negative). The methodological quality of the included articles was evaluated by two pulmonologists, A Shiroshita and Y Tanaka, using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (11). If there were discrepancies between the assessments by the two reviewers, then they were resolved by discussion or consultation with another pulmonologist (K Nakashima).
Data analysis
Data were collected regarding the sensitivity and specificity (including the 95% confidence interval) of each BFL serologic assay. To assess for pooled sensitivity and specificity, a bivariate random-effects model was used, and a hierarchical summary receiver operating characteristic (HSROC) curve was rendered to synthesize information on the diagnostic accuracy of the antibody-based tests. To assess the overall quality of the evidence, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach was used with independent review, as mentioned above. Although subgroup analysis targeting unhealthy people and those with acute versus chronic BFL was planned in the protocol, information on the sensitivity and specificity of patients without healthy controls was lacking; thus, the subgroup analysis was not feasible.
All analyses were completed using Diagnostic Test Accuracy Meta-Analysis software, version 1.21 (12) and RevMan software, version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration).
Results
Search results and study characteristics
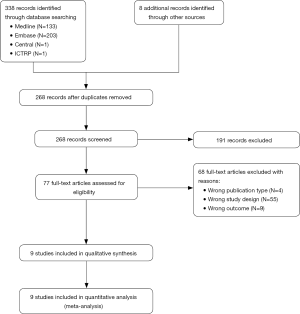
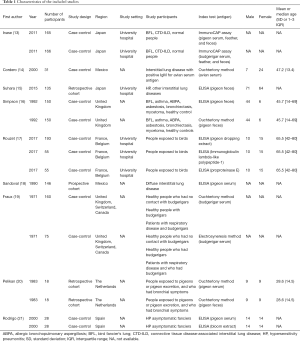
A total of 346 citations were identified up to January 21, 2019. Of these, 78 duplicate articles were removed, and the titles and abstracts of 268 articles were screened. Thus, 77 full-text manuscripts were acquired, of which four articles were excluded due to publication type (three case series and one review article), 55 articles were excluded due to the study design, and nine articles were excluded due to outcome measures. Finally, nine articles were included in this meta-analysis (Figure 1) (13-21). The study characteristics are summarized in Table 1 and Table S2. Notably, insufficient data pertaining to the study setting or BFL subtype were available to perform subgroup analysis of these parameters. Further, participant characteristics varied largely across the included studies.
Full table
Full table
Study quality
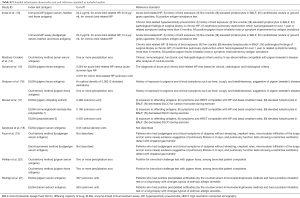
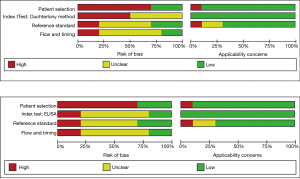
The risk of bias and the applicability of each antibody were evaluated using QUADAS-2 (Figure 2). Of note, almost all of the included studies demonstrated a low applicability risk and were associated with a high or uncertain risk of bias. Further, the studies were unclear regarding the processes used to diagnose BFL, lacked index test cut-offs, and did not prespecify diagnostic reference standards or indicate whether physicians made a diagnosis without knowledge of an index test. Moreover, most of the studies lacked information on the workflow and timing of diagnostic testing and had a high risk of bias.
Diagnostic accuracy of IgG antibodies
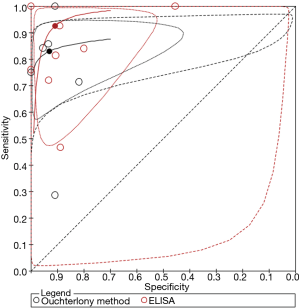
The pooled sensitivity and specificity of each specific IgG antibody was calculated using a bivariate mixed-effect model. Accordingly, the respective sensitivity and specificity of each method were as follows: Ouchterlony method, 82.9% (95% confidence interval, 71.1–90.5%) and 93.0% (95% confidence interval, 74.4–98.4%); ELISA method, 92.5% (95% confidence interval, 71.3–98.4%) and 90.8% (95% confidence interval, 72.1–97.4%); electrosyneresis method, 90.0% (95% confidence interval, 55.5–99.7%) and 84.6% (95% confidence interval, 73.5–92.4%); ImmunoCAP assay 43.5% (95% confidence interval, 35.3–52.1%) and 100% (95% confidence interval, 0–100%). Further, the diagnostic accuracy of the Ouchterlony and ELISA methods were visually evaluated using rendered HSROC curves (Figure 3), which demonstrated that the 95% prediction region was imprecise, as the Ouchterlony method had a higher sensitivity and the ELISA method had a higher specificity.
Subgroup analysis was performed using a bivariate mixed-effect model according to the type of BFL (acute or chronic); only two studies and three types of index tests (one ELISA and two ImmunoCAP assay) were included in this analysis. As such, both the sensitivity and specificity were higher in acute relative to chronic BFL (sensitivity 100% versus 98.0%, specificity 89.3% versus 55.1%, respectively).
Finally, the overall quality of evidence was assessed for the Ouchterlony and ELISA methods, using the GRADE approach (Tables 2,3).

Full table

Full table
Conclusions
In this systematic review, the sensitivity and specificity were estimated for four specific-IgG serologic assays used in diagnosing BFL because accurate antigen detection is crucial for treating this disease (22). Accordingly, the sensitivity and specificity of the methods were ranked as follows: sensitivity, ELISA > electrosyneresis method > Ouchterlony method > ImmunoCAP; specificity, ImmunoCAP method > electrosyneresis method > Ouchterlony method > ELISA. However, the overall quality of evidence was low due to a high risk of bias, indirectness, and imprecision associated with the included studies. Moreover, as the number of publications describing the use of electrosyneresis and ImmunoCAP assays was limited, the assessment of the diagnostic accuracy of these tests lacks precision. Thus, this analysis focused on comparing the Ouchterlony and ELISA methods.
According to this analysis, the Ouchterlony method of diagnosing BFL was associated with high specificity, suggesting that this method is a useful diagnostic tool in cases where physicians suspect BFL based on exposure history and radiological evidence of interstitial pneumonia. Further, the use of the Ouchterlony method may decrease unnecessary antigen avoidance, which is a first-line treatment strategy for BFL. Indeed, removing all bird, or bird-related items (such as feather bedding) is recommended, although some patients require a complete change of environment as the antigen can remain in the environment even after cleanup (23). Antigen avoidance can be emotionally and financially challenging to patients; thus, using a diagnostic method with high specificity may decrease the rate of false positives and the associated stress to patients and their families.
However, the Ouchterlony method is associated with disadvantages, including the fact that it is a qualitative method that is both time consuming and technically challenging. Thus, it is associated with poor reproducibility. Additionally, relatively large quantities of antigens are required to visualize a precipitating line (24). Unfortunately, information was not available regarding whether the method was performed by trained laboratory staff with standardized procedures. Future studies should include detailed workflows, including staff qualifications, to address this issue.
In contrast, the sensitivity of ELISA testing was higher than that of the Ouchterlony method, suggesting that ELISA may be a useful screening tool when BFL is suspected. The greater sensitivity may be explained by the consideration that ELISAs do not depend on visualization of a precipitate line, whereas the Ouchterlony method does. Further, ELISA testing is relatively fast and simple (25). Based on the publications included in this review, ELISA is also used more frequently for diagnosing BFL than the Ouchterlony method. In particular, Suhara et al. used ELISA for diagnosing acute and chronic BFL (15). As such, the researchers determined that the test exhibited higher sensitivity and specificity in diagnosing acute BFL, relative to chronic BFL. In acute BFL, humoral immunity is believed to dominate the immune response, while cell-mediated immunity dominates in chronic BFL (26). Thus, ELISA may be a more useful screening tool for acute rather than for chronic BFL.
Unfortunately, only a limited number of studies have utilized the electrosyneresis (n=1) or ImmunoCAP assay (n=1) methods for diagnosing BFL, which is likely due to the fact that these are relatively new antibody-based detection methods. However, according to this analysis, the electrosyneresis method had high sensitivity and specificity, whereas the ImmunoCAP assay had low sensitivity and high specificity. Notably, other investigators have suggested that the electrosyneresis method could be expected to support the diagnosis of HP caused by mold antigens as well (27), which was not accounted for by the single electrosyneresis publication included in this systematic review (19). For the ImmunoCAP assay, the only study included in this review was conducted in Japan (15). Concerning the high risk of bias in these tests, interpretation without knowledge of other test results will be needed for future cohort studies.
This systematic review has several limitations. Firstly, the number of included studies was small, and the heterogeneity was high. This contributed to unstable prediction regions for both the Ouchterlony and ELISA methods, suggesting that the mean observed sensitivity and specificity may change following the publication of additional studies in the future. Secondly, most of the studies had a high risk of bias because they were designed as case-control studies, and the processes used for BFL diagnosis were not described in detail. Moving forward, future studies should be completed as prospective cohort studies with larger sample sizes. It is also important to note that the antigenic component of the avian protein is currently unknown (23) and that identification of the antigenic peptide will likely be associated with a significant increase in diagnostic accuracy.
According to this systematic review and meta-analysis, the Ouchterlony method for BFL diagnosis is likely associated with high specificity, whereas the ELISA method likely has high sensitivity. Unfortunately, the diagnostic value of the electrosyneresis method and ImmunoCAP assay could not be accurately assessed at this moment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Costabel U, Bonella F, Guzman J. Chronic hypersensitivity pneumonitis. Clin Chest Med 2012;33:151-63. [Crossref] [PubMed]
- Bang KM, Weissman DN, Pinheiro GA, et al. Twenty-three years of hypersensitivity pneumonitis mortality surveillance in the United States. Am J Ind Med 2006;49:997-1004. [Crossref] [PubMed]
- Selman M. Hypersensitivity pneumonitis: a multifaceted deceiving disorder. Clin Chest Med 2004;25:531-47. [Crossref] [PubMed]
- Morisset J, Johannson KA, Jones KD, et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med 2018;197:1036-44. [Crossref] [PubMed]
- Vasakova M, Morell F, Walsh S, et al. Hypersensitivity pneumonitis: perspective in diagnosis and management. Am J Respir Crit Care Med 2017;196:680-9. [Crossref] [PubMed]
- Richerson HB, Bernstein IL, Fink JN, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis: Report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol 1989;84:839-44. [Crossref] [PubMed]
- Lacasse Y, Selman M, Costabel U, et al. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med 2003;168:952-8. [Crossref] [PubMed]
- Quirce S, Vandenplas O, Campo P, et al. Occupational hypersensitivity pneumonitis: as EAACI position paper. Allergy 2016;71:765-79. [Crossref] [PubMed]
- Schuyler M, Cormier Y. The diagnosis of hypersensitivity pneumonitis. Chest 1997;111:534-6. [Crossref] [PubMed]
- Fink JN, Schlueter DP, Sosman AJ, et al. Clinical survey of pigeon breeders. Chest 1972;62:277-81. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Freeman SC, Kerby CR, Patel A, et al. Development of an interactive web-based tool to conduct and interrogate meta-analysis of diagnostic test accuracy studies. BMC Med Res Methodol 2019;19:81. [Crossref] [PubMed]
- Inase N, Unoura K, Miyazaki Y, et al. Measurement of bird specific antibody in bird-related hypersensitivity pneumonitis. Nihon Kokyuki Gakkai Zasshi 2011;49:717-22. [PubMed]
- Martínez-Cordero E, Aguilar DE, Retana VN. IgM antiavian antibodies in sera from patients with pigeon breeder's disease. J Clin Lab Anal 2000;14:201-7. [Crossref] [PubMed]
- Suhara K, Miyazaki Y, Okamoto T, et al. Utility of immunological tests for bird-related hypersensitivity pneumonitis. Respir Investig 2015;53:13-21. [Crossref] [PubMed]
- Simpson C, Shirodaria PV, Evans JP, et al. Comparison of immunodiffusion and enzyme linked immunosorbent assay in the detection of abnormal antibodies in pigeon breeder's disease. J Clin Pathol 1992;45:490-3. [Crossref] [PubMed]
- Rouzet A, Reboux G, Dalphin JC, et al. An immunoproteomic approach revealed antigenic proteins enhancing serodiagnosis performance of bird fancier's lung. J Immunol Methods 2017;450:58-65. [Crossref] [PubMed]
- Sandoval J, Bañales JL, Cortés JJ, et al. Detection of antibodies against avian antigens in bronchoalveolar lavage from patients with pigeon breeder's disease: usefulness of enzyme-linked immunosorbent assay and enzyme immunotransfer blotting. J Clin Lab Anal 1990;4:81-5. [Crossref] [PubMed]
- Faux JA, Wide L, Hargreave FE, et al. Immunological aspects of respiratory allergy in budgerigar (Melopsittacus undulatus) fanciers. Clin Allergy 1971;1:149-58. [Crossref] [PubMed]
- Pelikan Z, Schot JD, Koedijik FH. The late bronchus-obstructive response to bronchial challenge with pigeon feces and its correlation with precipitating antibodies (IgG) in the serum of patients having long-term contact with pigeons. Clin Allergy 1983;13:203-11. [Crossref] [PubMed]
- Rodrigo MJ, Benavent MI, Cruz MJ, et al. Detection of specific antibodies to pigeon serum and bloom antigens by enzyme linked immunosorbent assay in pigeon breeder's disease. Occup Environ Med 2000;57:159-64. [Crossref] [PubMed]
- Holland AE, McDonald CF. Pulmonary rehabilitation and interstitial lung disease. Thorax 2009;64:548. [PubMed]
- Craig TJ, Hershey J, Engler RJ, et al. Bird antigen persistence in the home environment after removal of the bird. Ann Allergy 1992;69:510-2. [PubMed]
- Longbottom JL, Austwick PK. Antigens and allergens of Aspergillus fumigatus. I. Characterization by quantitative immunoelectrophoretic techniques. J Allergy Clin Immunol 1986;78:9-17. [Crossref] [PubMed]
- Douillard JY, Hoffman T, Herberman RB. Enzyme-linked immunosorbent assay for screening monoclonal antibody production: use of intact cells as antigen. J Immunol Methods 1980;39:309-16. [Crossref] [PubMed]
- Barrera L, Mendoza F, Zuñiga J, et al. Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 2008;177:44-55. [Crossref] [PubMed]
- Fenoglio CM, Reboux G, Surde B, et al. Diagnostic value of serum precipitins to mould antigens in active hypersensitivity pneumonitis. Eur Respir J 2007;29:706-12. [Crossref] [PubMed]