
Optimized stereoelectroencephalography-guided radiofrequency thermocoagulation in the treatment of patients with focal epilepsy
Introduction
Epilepsy is a severe health disorder affecting people of all ages with high prevalence worldwide. The introduction of new antiepileptic drugs has yielded notable effects in recent decades, yet there are still approximately 30% of patients with drug-resistant epilepsy (1). Currently, epilepsy surgery is widely accepted as a highly effective treatment for drug-resistant epilepsy. The last 20 years have witnessed a remarkable increase in the number of patients who have undergone surgery for drug-resistant epilepsy.
Classically, open surgical resection of the epileptogenic zone is the preferred procedure, with high rates of seizure-free outcomes. However, concerns about the impact of open surgery on brain function have driven considerable interest in less invasive techniques as an alternative to open surgery. Less-invasive treatment strategies, including laser interstitial thermotherapy, stereotactic radiosurgery, and high-intensity focal ultrasound, have been investigated over the years in an effort to reduce the risk of neurological and neuropsychological impairment by sparing functional tissue (2,3). These minimally invasive interventions also lead to less suffering, shorter hospitalization, better preservation of cerebral function, and reduced surgical complications (4,5).
Clinically, invasive EEG is indicated for approximately 30% of patients suffering from drug-resistant focal seizures in presurgical evaluation because of insufficient information from noninvasive investigation. Stereoelectroencephalography (SEEG), which was conceived by Bancaud and Talairach in the 1960s, offers a unique means of exploring the pathophysiologic process and accurately mapping the epileptogenic network through the analysis of the anatomo-electroclinical correlations. SEEG-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) has been reported since a 2004 feasibility study, initially intended to alleviate seizures (6). This technique allows ablation of the epileptogenic foci directly through the recording electrodes with electroclinical evidence. Due to its ability to achieve relatively well-circumscribed lesions and perform stimulation and recording at the same time as well as real-time monitoring of the impedance, the number of studies reporting SEEG-guided RF-TC for drug-resistant focal epilepsy has considerably increased in recent years (7-16). Overall, the basic principle of SEEG-guided RF-TC involves selectively destroying epileptogenic foci or critical nodes in epileptogenic networks, which can be considered a palliative approach. In addition, the curative effect of this procedure has been proposed in patients with epilepsy related to gray matter nodular heterotopy (9,12,17). Currently, the seizure outcome varies widely across studies, and the factors associated with efficacy remain elusive. Herein, we attempt to address the technical details and the selection of patients for this procedure based on the literature as well as our experiences to shed light on optimized SEEG-guided RF-TC in the treatment of patients with focal epilepsy.
Optimization of SEEG-guided RF-TC parameters to ablate seizure onset zone
The primary purpose of SEEG-guided RF-TC is to disrupt the epileptogenic network based on the SEEG findings, thereby reducing the seizure burden or achieving seizure-free status. The identification of seizure-onset and early propagation areas are fundamental to the treatment. Importantly, various protocols and RF-TC parameters have been tested across epilepsy centers to date.
High power levels of 6–8.32 W have been typically adopted in European epilepsy centers. With these power levels that could result in impedance collapse, it was believed that the range of RF-TC could be largest at maximum power, and the resulting lesions were reported to be 5–7 mm in diameter (6). In addition to power, attention was paid to lengthening the total duration of coagulation in the report by Bourdillon et al., in which increasing the voltage of the delivered current until these parameters spontaneously collapsed provided larger lesions than using fixed parameters (11). Regarding the constitution of the dipole field, coagulation should be performed via a pair of consecutive contacts within a single electrode according to French guidelines (18). Of note, Staudt et al. attempted to use different power levels in egg albumen and found that when 3 W was delivered, the lesioning size could be increased by increasing the effective duration of the power delivery. They also showed that the lesioning field obviously increased through a pair of contacts from different electrodes in a parallel arrangement (19).
Among these observations, earlier protocols from European centers have been widely adopted in clinical usage. The seizure outcomes of reported clinical cases are summarized as follows.
Lyon’s protocol
In 2004, SEEG-guided RF-TC was first reported in 20 patients with focal epilepsy in a study by Guenot et al. (6). in Lyon’s group. In their preliminary study, coagulations were created between two contiguous contacts by delivering 6 W within 40–50 s and thereby acquiring an estimated temperature of 78–82 °C. With these parameters, lesions of 5 to 7 mm in diameter could be reliably produced. No permanent neurologic or cognitive impairment was observed in their series, and 15% of the patients were seizure-free, while 40% experienced a seizure frequency reduction of at least 80%. Seven years later, with the same parameters and technical details, Guenot et al. reported their experiences among 41 patients, showing 48.7% of them with at least 50% seizure frequency reduction (8). Up to 162 patients were enrolled over ten years after the initiation of their protocol, and 7% of patients were seizure-free (14). Of note, the same technical protocol was employed in 14 patients with malformation of cortical development (MCD) at another center, and 64% of the patients obtained more than 50% seizure reduction, of whom 43% patients were seizure-free at an average follow-up of 41.7 months (10).
Milano’s protocol
In a study focused on the SEEG-guided RF-TC of gray-matter nodular heterotopy by Cossu et al. in Milano’s group, 8.32 W was delivered within 40–50 s to reach an estimated tissue temperature of 78–82 °C. In this study, five patients were enrolled, 4 of whom were seizure-free at an average follow-up of 33.5 months (9). In another series presented by Cossu et al., 89 patients with epileptogenic zones were treated with the same parameters. Among them, 16 patients were seizure-free (16). For patients with periventricular nodular heterotopia (PNH), the same protocol was proven to be efficacious in 76% of the patients (12).
The early experiences of RF-TC in Xuanwu Hospital
Selective disruption of the epileptogenic network is effective if the network can be delineated based on the analysis of anatomo-electroclinical correlations. However, incomplete mapping of the circuit is inevitable. Alternatively, ablation of small, deeply seeded or well-demarcated epileptogenic foci can be speculated. Based on the experiences from the above epilepsy centers, we further examined ways to produce extended thermocoagulative lesions. Therefore, a targeted multielectrode RF-TC procedure was addressed experimentally in our center, which can be defined as optimized cross-bonding RF-TC (15,20).
In vitro experiments performed on egg albumen helped to confirm the feasibility of our optimized cross-bonding RF-TC. We also noticed that the volume and shape of cross-bonding RF-TC in egg albumen were not very stable or repeatable. Considering that the brain tissue is solid rather than liquid, experiments in a solid phantom were considered to be more appropriate for imitating the cerebral environment. Therefore, using a more uniform solid phantom of polyacrylamide gel and a more quantitative approach, we observed that an extended confluent volume of coagulation could be created and a temperature between 60–78 °C could be achieved by integrating more contacts from multiple different electrodes together and delivering 3 W for 150 s. Moreover, the effective distance between contacts was found to be approximately 7 mm (Figure 1). Of note, the working parameters of clinical RF-TC cannot be decided based on In vitro experiments because the brain is not isotropic and is composed of different tissues, such as gray matter, white matter, vessels, CSF and lesions. It appeared that the suggested RF-TC power used in the human brain still should be reduced somehow compared with the polyacrylamide gel, or the impedance would be much more likely to collapse.

Regarding the protocol, stimulation of the suspected target to determine whether a habitual seizure could be provoked is also crucial for improving the seizure outcome, in line with the French guidelines (18). Additionally, we evaluated the function of all possible targets by bipolar electrical stimulation during a video-SEEG recording session before the ablation procedure, and a lesioning process without anesthesia was required if possible. We commonly heated the target to 42–43 °C by using a power of 2 W delivered for 30 s to observe whether neurological deficits would occur prior to the irreversible ablation. It is recommended to monitor SEEG activity after the coagulation process.
Refinement in patient selection for SEEG-guided RF-TC
According to the French guidelines, SEEG-guided RF-TC has been suggested to be a therapeutic alternative when there is contraindication to surgical resection. In particular, deep heterotopic nodules and hypothalamic hamartoma (HH) are recommended to avoid the high risk of open surgery (17,21).
Moreover, SEEG-guided RF-TC might be optional for patients with other types of epilepsy, such as focal epilepsy due to focal cortical dysplasias in the eloquent cortex or mesial temporal lobe epilepsy.
Deeply seeded epileptic lesions: hamartomas and NH
HH is a peculiar clinico-radiological syndrome with an incidence ranging from 1 in 100,000 to 1 in 1,000,000 (22). HH is a congenital, ectopic mass lesion typically arising from the tuber cinereum, which can be small, large, pedunculated, or sessile (23). HH is associated with a range of neurological symptoms, neuropsychiatric comorbidities, and endocrine disorders, which are mainly influenced by neuroanatomical features of the lesion (24-27). In particular, epilepsy has been recognized as the hallmark clinical picture of HH characteristically presenting with quite stereotyped gelastic and dacrystic seizures as well as with combinations of other types of seizures (28-30). Epilepsy related to HH is often drug-resistant, and studies defining the intrinsic epileptogenicity of HH in the 1990s confirmed the rationale of surgical treatment (30,31). Open surgery of HH could result in seizure-free status in approximately 50% of cases, yet some patients suffered from short-term memory disturbances and deep-seated ischemic injuries after the craniotomy (32-34). Apart from these complications, this surgical intervention has been typically associated with incomplete hamartoma resection (32-34). Given the risks, less invasive lesioning techniques for HH, including RF-TC, radiosurgery, and deep brain stimulation, have been proposed (15,35,36).
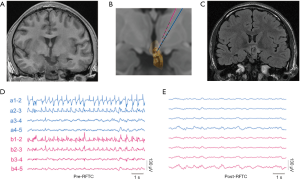
In the epilepsy center of our hospital, optimized cross-bonding RF-TC was performed on HH-associated drug-resistant epilepsy with high effectiveness and low complication rates (15). In brief, SEEG electrodes were implanted into the HH. The numbers of electrodes targeting the hamartoma ranging from 1 to 4 depending on the anatomic location and size of the HH. Figure 2 illustrates an example patient with HH who underwent SEEG-guided RF-TC at our hospital. This 19-year-old man had daily drug-resistant gelastic seizures, sometimes followed by tonic-clonic seizures. MRI revealed a sessile HH. The SEEG study showed continuous interictal epileptiform discharges in the hamartoma before RF-TC. Spiking activity following the application of RF-TC to the HH contacts completely disappeared. Currently, the patient is seizure-free 16 months after the procedure.
NH is a congenital disorder of neuronal migration characterized by ectopic nodules of neurons frequently located along the walls of the lateral ventricles (PNH) or masses of gray matter within the deep white matter (subcortical heterotopia) (37). Seizures related to NH are usually drug-resistant, and it is difficult for surgery to achieve a radical effect due to the unclear role of the heterotopic lesion in the epileptogenic network (38-40). According to the experiences reported by Cossu et al., SEEG-guided RF-TC is a curative first-line therapy in patients with epilepsy related to NH. Two-thirds of such patients achieved sustained seizure freedom after SEEG-guided RF-TC based on this report, so the implantation schedule of SEEG in this kind of case is now aimed at an intensive sampling of the epileptogenic network for effective thermocoagulation (17).
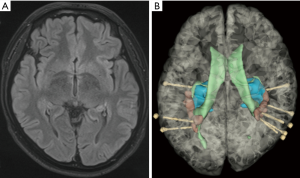
Figure 3 illustrates an example patient with PNH in our hospital. To define the roles of the heterotopic and normotopic cortexes in the epileptogenic network in this 23-year-old man, implantation of depth electrodes was scheduled. Bilateral heterotopic lesions and right mesial temporal structures were involved in the epileptogenic network, which was confirmed by SEEG recordings. The patient was seizure-free 2 years after multitargeting thermocoagulation of the bilateral periventricular lesions and right mesial temporal structures.
MTLE with unilateral hippocampal sclerosis
MTLE with hippocampal sclerosis has been drawing considerable interest due to its high prevalence and being the most common indication for surgery in adults with drug-resistant epilepsy. Anterior-mesial temporal lobectomy by open resection or selective amygdalohippocampectomy is an effective treatment for drug-resistant MTLE-HS patients (41-43). Prospective, randomized trials demonstrate that seizure-free rates are significantly higher in surgically treated patients compared to those given the best medical therapy. Surgical resection, however, entails risks for injury of normal brain tissue and accompanying neuropsychological deficits, especially cognitive impairments (44,45). A postoperative decline in episodic memory performance following ATL surgery has been observed in 25–50% of these patients (46). Thus, in recent years, various minimally invasive approaches have been explored for MTLE by maximizing seizure control and minimizing cognitive impairments. Among them, laser interstitial thermal therapy (LiTT) has been investigated with a relatively satisfactory outcome (2,5). However, LiTT is not universally available, and its indications, safety, and efficacy need further evaluation. SEEG-guided RF-TC for the treatment of MTLE with hippocampal sclerosis is attractive. Nevertheless, Lyon’s group found that SEEG-guided RF-TC was not as effective as anterior temporal lobectomy (ATL) since none of the patients was seizure-free in the RF-TC group, in contrast to the seizure-free rate of 75.5% among the ALT group (13).
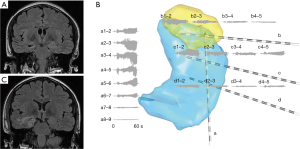
To optimize SEEG-guided RF-TC as a potential minimally invasive treatment option for MTLE, we recently conducted SEEG-guided RF-TC with 3D cross-bonded electrode contacts to maximize RF-TC efficacy in patients with MTLE-HS. In brief, SEEG electrode implantation was designed to form a focal stereoarray to sample mesial temporal structures widely and guide the subsequent RF-TC. Instead of delivering RF-TC to a predetermined location in the sclerotic hippocampus via one electrode, a targeted multielectrode RF-TC procedure was conducted based on the identification of the ictal onset zone or zones by SEEG. Thus, the modified approach produced extended thermocoagulative lesions similar to a selective amygdalohippocampectomy involving the amygdalohippocampal complex, subiculum, and part of the entorhinal cortex (Figure 4). Our preliminary observations demonstrated a relatively favorable seizure outcome and no surgical complications in patients with unilateral HS (20).
Focal cortical dysplasias in eloquent cortex
Patients with seizures originating primarily from the eloquent cortex pose a challenge because the removal of such epileptic tissue carries a high risk of specific and irreversible neurological functional deficits. In particular, permanent neurological deficits can occur following resection of primary functional areas, including the primary motor, sensory, visual cortex, Broca’s area, and Wernicke’s area (47,48). Therefore, the exploration of alternative therapeutic methods for drug-resistant epilepsy patients with epileptogenic zones involving the eloquent cortex continues to be a topic of intensive study.
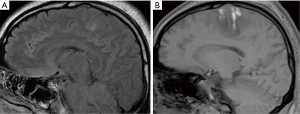
Confronted with this challenge, we performed SEEG-guided RF-TC in refractory focal epilepsy patients with clinically well-demarcated epileptic foci in the eloquent cortex. Figure 5 illustrates one representative patient with a lesion located in the right paracentral lobule who was treated with SEEG-guided RF-TC. This 26-year-old man had daily refractory seizures manifesting as ictal tonic posturing of the left leg. A transient weakness of the left foot completely disappeared within a few weeks. The patient was seizure-free 2 years after the procedure.
Conclusions
Currently, SEEG-guided RF-TC is increasingly recognized as a treatment option for patients with focal drug-resistant seizures. Optimization of SEEG-guided RF-TC parameters and refinements in patient selection for SEEG-guided RF-TC are essential to attain favorable efficacy. Although accumulating evidence has shown that SEEG-guided RF-TC is a promising option for patients with refractory seizures, the indications for SEEG-guided RF-TC are still being investigated. More caution should be taken in the selection of patients who can benefit significantly from such a novel intervention. In particular, the application of SEEG-guided RF-TC in patients with uncertain epileptogenic foci is unreasonable.
Future investigations of SEEG-guided RF-TC are needed in some aspects. Since parameters and protocols among epilepsy centers worldwide vary, consensus is necessary to guide SEEG-guided RF-TC in clinical practice. Technically, developing new devices for RF-TC that provide intraoperative control over lesion temperature and real-time lesion visualization would help produce ablated volumes as expected with better outcomes. Importantly, randomized controlled trials with long-term follow-up are needed to compare the efficacy of SEEG-guided RF-TC with conventional methods in the future.
Acknowledgments
The authors would like to thank Xiaotong Fan for critical discussion and reading of the manuscript.
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 81871009, 81801288, 81571271, 81771398), National Key R&D Program of China (Grant No. 2016YFC0103909), and the Key Project of Beijing Science and Technology Commission (Capital characteristics, Grant No. Z161100000516008).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000;342:314-9. [Crossref] [PubMed]
- Shukla ND, Ho AL, Pendharkar AV, et al. Laser interstitial thermal therapy for the treatment of epilepsy: evidence to date. Neuropsychiatr Dis Treat 2017;13:2469-75. [Crossref] [PubMed]
- Sinha S, Danish SF. History and Technical Approaches and Considerations for Ablative Surgery for Epilepsy. Neurosurg Clin N Am 2016;27:27-36. [Crossref] [PubMed]
- Chang EF, Englot DJ, Vadera S. Minimally invasive surgical approaches for temporal lobe epilepsy. Epilepsy Behav 2015;47:24-33. [Crossref] [PubMed]
- Gross RE, Mahmoudi B, Riley JP. Less is more: novel less-invasive surgical techniques for mesial temporal lobe epilepsy that minimize cognitive impairment. Curr Opin Neurol 2015;28:182-91. [Crossref] [PubMed]
- Guenot M, Isnard J, Ryvlin P, et al. SEEG-guided RF thermocoagulation of epileptic foci: feasibility, safety, and preliminary results. Epilepsia 2004;45:1368-74. [Crossref] [PubMed]
- Catenoix H, Mauguiere F, Guenot M, et al. SEEG-guided thermocoagulations: a palliative treatment of nonoperable partial epilepsies. Neurology 2008;71:1719-26. [Crossref] [PubMed]
- Guenot M, Isnard J, Catenoix H, et al. SEEG-guided RF-thermocoagulation of epileptic foci: a therapeutic alternative for drug-resistant non-operable partial epilepsies. Adv Tech Stand Neurosurg 2011;36:61-78. [Crossref] [PubMed]
- Cossu M, Fuschillo D, Cardinale F, et al. Stereo-EEG-guided radio-frequency thermocoagulations of epileptogenic grey-matter nodular heterotopy. J Neurol Neurosurg Psychiatry 2014;85:611-7. [Crossref] [PubMed]
- Catenoix H, Mauguiere F, Montavont A, et al. Seizures Outcome After Stereoelectroencephalography-Guided Thermocoagulations in Malformations of Cortical Development Poorly Accessible to Surgical Resection. Neurosurgery 2015;77:9-14; discussion 14-5. [Crossref] [PubMed]
- Bourdillon P, Isnard J, Catenoix H, et al. Stereo-electro-encephalography-Guided Radiofrequency Thermocoagulation: From In Vitro and In Vivo Data to Technical Guidelines. World Neurosurg 2016;94:73-9. [Crossref] [PubMed]
- Mirandola L, Mai RF, Francione S, et al. Stereo-EEG: Diagnostic and therapeutic tool for periventricular nodular heterotopia epilepsies. Epilepsia 2017;58:1962-71. [Crossref] [PubMed]
- Moles A, Guenot M, Rheims S, et al. SEEG-guided radiofrequency coagulation (SEEG-guided RF-TC) versus anterior temporal lobectomy (ATL) in temporal lobe epilepsy. J Neurol 2018;265:1998-2004. [Crossref] [PubMed]
- Bourdillon P, Isnard J, Catenoix H, et al. Stereo electroencephalography-guided radiofrequency thermocoagulation (SEEG-guided RF-TC) in drug-resistant focal epilepsy: Results from a 10-year experience. Epilepsia 2017;58:85-93. [Crossref] [PubMed]
- Wei PH, An Y, Fan XT, et al. Stereoelectroencephalography-Guided Radiofrequency Thermocoagulation for Hypothalamic Hamartomas: Preliminary Evidence. World Neurosurg 2018;114:e1073-8. [Crossref] [PubMed]
- Cossu M, Fuschillo D, Casaceli G, et al. Stereoelectroencephalography-guided radiofrequency thermocoagulation in the epileptogenic zone: a retrospective study on 89 cases. J Neurosurg 2015;123:1358-67. [Crossref] [PubMed]
- Cossu M, Cardinale F, Casaceli G, et al. Stereo-EEG-guided radiofrequency thermocoagulations. Epilepsia 2017;58 Suppl 1:66-72. [Crossref] [PubMed]
- Isnard J, Taussig D, Bartolomei F, et al. French guidelines on stereoelectroencephalography (SEEG). Neurophysiol Clin 2018;48:5-13. [Crossref] [PubMed]
- Staudt MD, Maturu S, Miller JP. Radiofrequency Energy and Electrode Proximity Influences Stereoelectroencephalography-Guided Radiofrequency Thermocoagulation Lesion Size: An In Vitro Study with Clinical Correlation. Oper Neurosurg (Hagerstown) 2018;15:461-9. [Crossref] [PubMed]
- Fan X, Shan Y, Lu C, et al. Optimized SEEG-guided radiofrequency thermocoagulation for mesial temporal lobe epilepsy with hippocampal sclerosis. Seizure 2019;71:304-11. [Crossref] [PubMed]
- Youngerman BE, Khan FA, McKhann GM. Stereoelectroencephalography in epilepsy, cognitive neurophysiology, and psychiatric disease: safety, efficacy, and place in therapy. Neuropsychiatr Dis Treat 2019;15:1701-16. [Crossref] [PubMed]
- Frazier JL, Goodwin CR, Ahn ES, et al. A review on the management of epilepsy associated with hypothalamic hamartomas. Childs Nerv Syst 2009;25:423-32. [Crossref] [PubMed]
- Freeman JL, Coleman LT, Wellard RM, et al. MR imaging and spectroscopic study of epileptogenic hypothalamic hamartomas: analysis of 72 cases. AJNR Am J Neuroradiol 2004;25:450-62. [PubMed]
- Arita K, Ikawa F, Kurisu K, et al. The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg 1999;91:212-20. [Crossref] [PubMed]
- Chan YM, Fenoglio-Simeone KA, Paraschos S, et al. Central precocious puberty due to hypothalamic hamartomas correlates with anatomic features but not with expression of GnRH, TGFalpha, or KISS1. Horm Res Paediatr 2010;73:312-9. [Crossref] [PubMed]
- Parvizi J, Le S, Foster BL, et al. Gelastic epilepsy and hypothalamic hamartomas: neuroanatomical analysis of brain lesions in 100 patients. Brain 2011;134:2960-8. [Crossref] [PubMed]
- Kerrigan JF, Ng YT, Chung S, et al. The hypothalamic hamartoma: a model of subcortical epileptogenesis and encephalopathy. Semin Pediatr Neurol 2005;12:119-31. [Crossref] [PubMed]
- Berkovic SF, Andermann F, Melanson D, et al. Hypothalamic hamartomas and ictal laughter: evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann Neurol 1988;23:429-39. [Crossref] [PubMed]
- Mullatti N, Selway R, Nashef L, et al. The clinical spectrum of epilepsy in children and adults with hypothalamic hamartoma. Epilepsia 2003;44:1310-9. [Crossref] [PubMed]
- Munari C, Kahane P, Francione S, et al. Role of the hypothalamic hamartoma in the genesis of gelastic fits (a video-stereo-EEG study). Electroencephalography & Clinical Neurophysiology 1995;95:154-60. [Crossref] [PubMed]
- Kuzniecky R, Guthrie B, Mountz J, et al. Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol 1997;42:60-7. [Crossref] [PubMed]
- Palmini A, Chandler C, Andermann F, et al. Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology 2002;58:1338-47. [Crossref] [PubMed]
- Ng YT, Rekate HL, Prenger EC, et al. Transcallosal resection of hypothalamic hamartoma for intractable epilepsy. Epilepsia 2006;47:1192-202. [Crossref] [PubMed]
- Feiz-Erfan I, Horn EM, Rekate HL, et al. Surgical strategies for approaching hypothalamic hamartomas causing gelastic seizures in the pediatric population: transventricular compared with skull base approaches. J Neurosurg 2005;103:325-32. [PubMed]
- Marras CE, Rizzi M, Villani F, et al. Deep brain stimulation for the treatment of drug-refractory epilepsy in a patient with a hypothalamic hamartoma. Neurosurgical Focus 2011;30:E4. [Crossref] [PubMed]
- Curry DJ, Raskin J, Ali I, et al. MR-guided laser ablation for the treatment of hypothalamic hamartomas. Epilepsy Res 2018;142:131-4. [Crossref] [PubMed]
- Barkovich AJ, Guerrini R, Kuzniecky RI, et al. A developmental and genetic classification for malformations of cortical development: update 2012. Brain 2012;135:1348-69. [Crossref] [PubMed]
- Dubeau F, Tampieri D, Lee N, et al. Periventricular and subcortical nodular heterotopia. A study of 33 patients. Brain 1995;118:1273-87. [Crossref] [PubMed]
- Li LM, Dubeau F, Andermann F, et al. Periventricular nodular heterotopia and intractable temporal lobe epilepsy: poor outcome after temporal lobe resection. Ann Neurol 1997;41:662-8. [Crossref] [PubMed]
- Pizzo F, Roehri N, Catenoix H, et al. Epileptogenic networks in nodular heterotopia: A stereoelectroencephalography study. Epilepsia 2017;58:2112-23. [Crossref] [PubMed]
- Wiebe S, Blume WT, Girvin JP, et al. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 2001;345:311-8. [Crossref] [PubMed]
- Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 2005;128:1188-98. [Crossref] [PubMed]
- Spencer SS, Berg AT, Vickrey BG, et al. Initial outcomes in the Multicenter Study of Epilepsy Surgery. Neurology 2003;61:1680-5. [Crossref] [PubMed]
- Lutz MT, Clusmann H, Elger CE, et al. Neuropsychological outcome after selective amygdalohippocampectomy with transsylvian versus transcortical approach: a randomized prospective clinical trial of surgery for temporal lobe epilepsy. Epilepsia 2004;45:809-16. [Crossref] [PubMed]
- Sherman EM, Wiebe S, Fay-McClymont TB, et al. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia 2011;52:857-69. [Crossref] [PubMed]
- Helmstaedter C, Kurthen M, Lux S, et al. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol 2003;54:425-32. [Crossref] [PubMed]
- Pilcher C, Meacham WF, Holbrook TJ. Partial excision of the motor cortex in treatment of jacksonian convulsions; results in 41 cases. Arch Surg 1947;54:633-43. [Crossref] [PubMed]
- Ostergard TA, Miller JP. Surgery for epilepsy in the primary motor cortex: A critical review. Epilepsy Behav 2019;91:13-9. [Crossref] [PubMed]


