
Use of home neuromuscular electrical stimulation in the first 6 weeks improves function and reduces pain after primary total knee arthroplasty: a matched comparison
Introduction
Procedural volumes for total knee arthroplasties (TKAs) have surged over the past few decades, with recent projections indicating that its growth will continue to rise (1-3). Part of this increase in volume can be attributed to the generally high rates of patient satisfaction associated with the procedure (4,5). However, recent studies have found that 20% or more of TKA patients have some level of post-operative dissatisfaction contributed in part by decreased range of motion, loss of function, and post-operative pain (6,7). Notably, high rates of quadriceps muscle weakness following the procedure have been reported (8-12). Full strength of the quadriceps muscle is critical for pain reduction and adequate range of motion. One post-operative modality that can help improve regaining strength of this muscle is NMES (13).
NMES is a neuromodulatory technique that strengthens muscles through the increased recruitment of type II muscle fibers (14-16). Multiple studies have indicated that its use can increase quadriceps muscle strength following TKA (11,12,17-19). In a recent randomized control trial consisting of 70 patients receiving NMES in addition to standard physiotherapy, Avramidis et al. reported significant increases in Oxford Knee scores (P=0.001), American Knee Society function scores (P=0.001), and SF-36 physical component scores (P=0.001) compared to standard physiotherapy modalities alone (19). However, other studies have indicated that the addition of NMES to post-operative physical therapy (PT) did not significantly change outcome measures such as SF-36 sores (P=0.3) or maximum voluntary isometric contractions (P>0.05) (20,21). Furthermore, to the authors’ knowledge, the use of NMES has not been evaluated in patients who received muscle stimulators for home use immediately after surgery. Therefore, given the lack of information regarding the home use of NMES and the inconsistency regarding the efficacy of NMES use, further studies are needed related to these topics.
Therefore, the purpose of this study was to assess the effects of supplemental NMES home use on patient recovery following primary TKA. Furthermore, this study aimed to compare early functional results to a matched control cohort that did not receive home NMES units following their TKA procedures.
Methods
Patient selection
Forty-one patients scheduled for primary TKA between April 2017 and January 2018 were identified as eligible for the study. All surgeries were performed by 2 surgeons and had similar post-surgical protocols of therapy. All patients received a posterior cruciate-retaining device, and all surgeries were performed using a midline parapatellar incision. All patients were discharged with a prescription for narcotic analgesics with a 3-week supply of 5 mg oxycodone to be taken as needed.
Of the 41 patients initially identified, 15 were excluded from the study. Three patients declined to participate and 3 had implantable spine stimulators or defibrillators. Three developed post-surgical complication (2 DVT, 1 post-surgical CVA). Six patients were unable to attend follow-up appointments. This resulted in 26 patients who were included for final analysis. A matched cohort (n=26) was generated from a pool of 176 patients who underwent TKA surgery between June 2015 to July 2016 and who did not receive home NMES devices. Twenty-five out of 26 were matched within 5 points for BMI and within 6 years for age. Remaining one patient was matched within 7 points of BMI. This yielded a total of 52 patients included for analysis (Table 1).
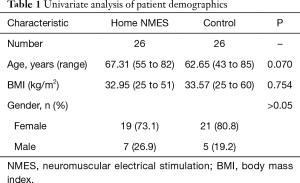
Full table
NMES device and protocol
Patients were fitted either 1 week before or within a week of surgery with a home NMES device (Cymedica Orthopedics, Phoenix, Arizona). The NMES device was Bluetooth enabled and was controlled by a smart phone (Figure 1). Patients were asked to use the device daily for 20 minutes, 3 times a day, for 6 weeks after surgery. As their ability to activate their quadriceps muscle increased, patients were encouraged to exercise by activating this muscle as they felt muscle stimulation to augment the effects of NMES. Initially, patients activated their quadriceps in the quadriceps-setting position performing voluntary isometric contraction in knee extended position as they felt muscle stimulation. As patients got stronger, they were encouraged to do isotonic knee extension exercises. Patients were also encouraged to increase the intensity of the stimulation every 3 to 4 minutes to overcome accommodation.

Outcome measures
Functional parameters were obtained for the study and control cohorts at approximately 6 weeks following surgery. These included timed up and go (TUG) time, single limb stance (SLS) time, time to ascend and descend one flight of stairs, quadriceps muscle lag, active and passive range of motion (ROM), 2-minute walking distance, and pain rating on a visual analog scale (VAS). This data was also obtained for the matching cohort.
Data analysis
Univariate analyses were performed with independent samples t-tests to compare outcomes: age, body mass index (BMI), resting pain, worst pain scores, quadriceps lag, TUG scores, SLS time, time to ascend and descend stairs, 2 minute walking, active range of motion in flexion and extension, and passive range of motion in flexion and extension between the two cohorts. Chi-square tests were used to compare any differences in sex between the cohorts. All statistical analyses were conducted using SPSS version 24 (Armonk, New York, USA). A P value of 0.05 was set as the threshold for statistical significance for the matched comparison.
Results
Patients in the home NMES cohort were found to have significantly better scores for quadriceps lag (P<0.001), TUG (P<0.001), time to ascend and descend one flight of stairs (P=0.001), 2-minute walking distance (P=0.001), and SLS time (P<0.001) (Table 2). Additionally, patients who received home NMES units experienced significantly lower resting pain (P<0.001) and lower worst reported pain scores (P<0.001) compared to the control cohort. Furthermore, there were a higher percentage of patients in the control cohort that could not use stairs reciprocally compared to the home NMES group (53.8% vs. 23.1%).
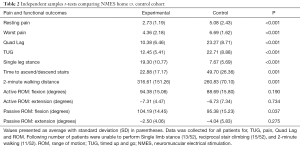
Full table
There was no significant difference in active or passive range of motion in the two groups except for passive flexion. However, while passive range of motion for flexion was statistically better in the home NMES group (P=0.037).
Discussion
The recognition of the importance of quadriceps muscle strength for optimal post-operative TKA outcomes has led to the development of a number of strength-enhancing modalities. Neuromuscular stimulation is one of these modalities and its use has continued to grow over recent years. However, it has yielded mixed results in the literature, and has primarily been studied when used during outpatient therapy rather than at home. Therefore, our study aimed to determine the effects of home NMES use following TKA on pain, function, and range of motion parameters. Our analysis demonstrated significant pain reduction both at rest and for worst reported pain scores, as well as improved function, with the use of a home-based NMES units for the sub-acute phase of the recovery when added to a standard therapy program. Patients walked and transferred safely as shown by improvements in TUG, reduction in quadriceps lag, and improvement in single limb support time. Furthermore, larger proportions of patients in the home NMES group were able to negotiate stairs reciprocally and faster than the matched control group.
Our study had some limitations. As a whole, the sample size for both the control and experimental cohorts was relatively small and not randomized. However, given that this was the first analysis that the authors are aware of that examined the use of home NMES units in a matched comparison, we believe this study size and attempts at matching the patients were sufficient. However, despite these limitations, we found that the addition of NMES home units to PT regiments resulted in improved pain and function compared to the PT regimen alone.
Our results are consistent with other studies examining the effects of NMES on post-operative outcomes (11,12,17-19). In a recent prospectively randomized control trial of 66 knees, Stevens-Lapsley et al. found that the use of NMES in addition to physical therapy resulted in significant improvements compared to controls receiving physical therapy alone at a mean of 3.5 weeks following TKA (17). Specifically, NMES addition resulted in significantly better 6-minute walk tests (P<0.001), stair climb tests (P=0.001), TUG tests (P=0.003), and quadriceps muscle strength scores (P<0.001) (17). Similarly, walking speed was found to improve by a mean of 8 minutes by Avramidis et al. at 6-week (P=0.0002) and 3-month (P=0.0001) measurements for patients receiving NMES compared to a control cohort (18).
On the contrary, some studies have found that NMES addition did not yield significantly improved results (20,21). Petterson et al. compared 45 TKA patients who underwent physical therapy with volitional strength training to 47 patients who received NMES in addition to this strength training (21). At 3- and 12-month follow-ups, the authors found no statistically differences for any outcome measures, including TUG tests, stair climb tests, 6-minute walk tests, and quadriceps muscle strength assessments (all P>0.08) (21). However, the NMES cohort began using their devices 4 weeks following the primary TKA and therefore may not reflect the efficacy of NMES use in the immediate post-surgical period as was done in the present study. Similarly, Levine et al. compared patients receiving therapist-managed physical therapy to those undergoing unsupervised ROM exercises at home with NMES and found no significant differences between the two groups regarding TUG tests (14 vs. 14 seconds), Knee Society Scores (KSS), or Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores (20). However, the inherent design of that study did not control for NMES use alone and therefore, other confounding factors may have played a role in the reported findings.
Conclusions
The use of NMES with post-operative therapy is a promising method for improving patient outcomes for TKA. Our analysis found both significant pain reduction as well as significantly improved function when home NMES units were used in addition to standard physical therapy. Specifically, patients receiving NMES had significantly higher single limb support times, increased efficiency of navigating stairs, reduced TUG test time, and reduced quadriceps lag. Future studies should continue to evaluate the efficacy of these home units in larger cohorts of patients in randomized fashion. Similarly, studies should examine if there are predictive factors that allow identification of those patients who would benefit the most from home NMES therapy. In summary our study showed that addition of home NMES therapy for 20 minutes 3 times daily in addition to standard physical therapy program leads to accelerated recovery of function with reduced pain in post TKA patient. Use of this modality may help in shortening recovery cycle of a post TKA patient and reduce overall economic burden of this procedure.
Acknowledgments
None.
Footnote
Conflicts of Interest: A Bhave is consultant to Cymedica Orthopedics. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This was an observational study with retrospective data analysis of identified patients. No patient consent or any other approvals were necessary for this study.
References
- Cram P, Lu X, Kates SL, et al. Total Knee Arthroplasty Volume, Utilization, and Outcomes Among Medicare Beneficiaries, 1991-2010. JAMA 2012;308:1227-36. [Crossref] [PubMed]
- Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455-60. [Crossref] [PubMed]
- Inacio MCS, Paxton EW, Graves SE, et al. Projected increase in total knee arthroplasty in the United States – an alternative projection model. Osteoarthritis Cartilage 2017;25:1797-803. [Crossref] [PubMed]
- Baker PN, van der Meulen JH, Lewsey J, et al. The role of pain and function in determining patient satisfaction after total knee replacement: data from the national joint registry for England and wales. J Bone Joint Surg Br 2007;89:893-900. [Crossref] [PubMed]
- Anderson JG, Wixson RL, Tsai D, et al. Functional outcome and patient satisfaction in total knee patients over the age of 75. J Arthroplasty 1996;11:831-40. [Crossref] [PubMed]
- Kurtz SM, Lau EC, Ong KL, et al. Which Hospital and Clinical Factors Drive 30- and 90-Day Readmission After TKA? J Arthroplasty 2016;31:2099-107. [Crossref] [PubMed]
- Khan M, Osman K, Green G, et al. The epidemiology of failure in total knee arthroplasty. Bone Joint J 2016;98-B:105-12. [Crossref] [PubMed]
- Johnson AW, Myrer JW, Hunter I, et al. Whole-body vibration strengthening compared to traditional strengthening during physical therapy in individuals with total knee arthroplasty. Physiother Theory Pract 2010;26:215-25. [Crossref] [PubMed]
- Ardali G. A daily adjustable progressive resistance exercise protocol and functional training to increase quadriceps muscle strength and functional performance in an elderly homebound patient following a total knee arthroplasty. Physiother Theory Pract 2014;30:287-97. [Crossref] [PubMed]
- Mizner RL, Petterson SC, Stevens JE, et al. Early Quadriceps Strength Loss After Total Knee Arthroplasty. J Bone Joint Surg Am 2005;87:1047-53. [Crossref] [PubMed]
- Ulrich SD, Bhave A, Marker DR, et al. Focused rehabilitation treatment of poorly functioning total knee arthroplasties. Clin Orthop Relat Res 2007.138-45. [PubMed]
- Bhave A, Mont M, Tennis S, et al. Functional Problems and Treatment Solutions After Total Hip and Knee Joint Arthroplasty. J Bone Joint Surg Am 2005;87:9-21. [PubMed]
- Chughtai M, Piuzzi N, Yakubek G, et al. Use of an App-Controlled Neuromuscular Electrical Stimulation System for Improved Self-Management of Knee Conditions and Reduced Costs. Surg Technol Int 2017;31:221-6. [PubMed]
- Walls RJ, McHugh G, O'Gorman DJ, et al. Effects of preoperative neuromuscular electrical stimulation on quadriceps strength and functional recovery in total knee arthroplasty. A pilot study. BMC Musculoskelet Disord 2010;11:119. [Crossref] [PubMed]
- Werner S, Arvidsson H, Arvidsson I, et al. Electrical stimulation of vastus medialis and stretching of lateral thigh muscles in patients with patello-femoral symptoms. Knee Surg Sports Traumatol Arthrosc 1993;1:85-92. [Crossref] [PubMed]
- Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol 2011;111:2399-407. [Crossref] [PubMed]
- Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Early Neuromuscular Electrical Stimulation to Improve Quadriceps Muscle Strength After Total Knee Arthroplasty: A Randomized Controlled Trial. Phys Ther 2012;92:210-26. [Crossref] [PubMed]
- Avramidis K, Strike PW, Taylor PN, et al. Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty. Arch Phys Med Rehabil 2003;84:1850-3. [Crossref] [PubMed]
- Avramidis K, Karachalios T, Popotonasios K, et al. Does Electric Stimulation of the Vastus Medialis Muscle Influence Rehabilitation After Total Knee Replacement? Orthopedics 2011;34:175. [Crossref] [PubMed]
- Levine M, McElroy K, Stakich V, et al. Comparing Conventional Physical Therapy Rehabilitation With Neuromuscular Electrical Stimulation After TKA. Orthopedics 2013;36:e319-24. [Crossref] [PubMed]
- Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum 2009;61:174-83. [Crossref] [PubMed]


