Pre-treatment Glasgow prognostic score and modified Glasgow prognostic score may be potential prognostic biomarkers in urological cancers: a systematic review and meta-analysis
Introduction
Urological cancers, mainly including bladder cancer (BC), prostate cancer (PC) and renal cell carcinoma (RCC), are common malignancies which bring heavy burden to human health. In 2018, the globally estimated new cases for BC, PC and RCC were 549,393, 1,276,106, and 403,262, respectively (1). Although improved survival outcomes have been made due to the development of surgical-centered comprehensive treatment, the prognosis of advanced patients is still unsatisfactory because of recurrence and metastasis. Additionally, only 25% patients could benefit from immunotherapy (2). In terms of BC, the 5-year survival was only 5.4% for distant metastasis (3). As for RCC, recurrence occurred in one-third surgery patients (4). Hence, it may be of great importance to investigate prognostic factors for survival and recurrence in urological cancers, which could play a critical role in clinical decision.
Recently, accumulating evidence emerged on the prediction of tumor recurrence and survival using clinical parameters. In various solid tumors, prognostic value of systemic inflammatory response (SIR) had already been proved, and many studies supposed that SIR might promote tumor growth based on previous extracellular matrix enzymes, growth factors or proangiogenic factors in tumor microenvironment. Also, inflammatory cytokines could activate cancer stem cell pathway, which was proved to promote tumor invasion and development. Furthermore, the nutritional status was also verified to be closely associated with survival outcomes. Previous studies (5,6) found that a low pre-treatment prognostic nutritional index (PNI) was related to a worse prognosis in many types of cancer. In 2009, Karl et al. (7) evaluated the nutritional status of 897 urologic patients by using the Nutritional Risk Screening (NRS) 2002, and they found malnutrition could increase the risk of malignant disease.
Glasgow prognostic score (GPS)/modified GPS (mGPS), combination of pre-treatment albumin and C-reaction protein (CRP) levels, are two different concepts that have minor discrepancy in the definition of Score 1. Recently, both GPS and mGPS showed great value in predicting survival outcomes of various cancer types (8-10). Certainly, a lot of articles has explored the specific role of GPS or mGPS in urological cancers. As for BC, Miyake (11) and Wuethrich (12) demonstrated that higher pre-treatment mGPS/GPS was strongly associated with poorer overall survival (OS), and similar conclusions were drawn in RCC (13,14) and PC (15). However, Ferro (16) reported that pre-treatment mGPS could not be a predictive tool for OS and cancer specific survival (CSS). Another study (17) conducted by Cho supported this result. Additionally, debates still existed on the specific role of GPS/mGPS in urological cancers because of the differences in sample size, study design and the intermixed use of terms (GPS and mGPS). Hence, we performed this study to clarify the relationship between GPS/mGPS and prognosis of patients with urological cancers. To our best knowledge, this is the first meta-analysis to estimate the prognostic role of pre-treatment GPS/mGPS in urological cancers, which could provide clinical guidance in the future due to the lack of strong evidence guiding the clinical application of GPS/mGPS in urological cancers.
Methods
Search strategy
In order to investigate the relationship between mGPS/GPS, we searched relevant articles from public online databases including PubMed, Web of Science and Embase comprehensively, up to May 30th 2019. Text words and Medical Subject Headings (MeSH) terms were combined: (“modified Glasgow prognostic score” or “Glasgow prognostic score” or “GPS” OR “mGPS”) and (“urological/ urothelial tumor/cancer” or “prostate cancer” or “bladder cancer” or “renal cell cancer” or “upper tract urothelial carcinoma” or “penile cancer”) and (“progress” or “survival” or “outcome” or “prognosis” or “recurrence”). This study was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (18) and only English references were included in the selection process.
Inclusion/exclusion criteria and definitions
Relevant studies were finally included should meet the following criteria: (I) case control or cohort studies, (II) patients were diagnosed with urothelial carcinomas with histopathological results, (III) related endpoints were explored (such as: OS, progress-free survival (PFS), recurrence-free survival (RFS), CSS, disease-free survival (DFS), disease-specific survival (DSS) and corresponding data were present in the form of hazard ratios (HRs) with 95% confidence interval (CIs) in the articles. Meanwhile, articles should be excluded when meet any of the following criteria: (I) case report, letter or review, (II) accurate data were lacked, (III) simple description with no further analysis or key information.
The GPS score was defined as described before (19): patients with both elevated CRP (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) assigned a score of 2, while those with none or one abnormality were assigned scores of 0 and 1, respectively. The only difference between mGPS and GPS was that hypoalbuminemic patients without elevated CRP were assigned a score of 0 in mGPS score system.
Data extraction and quality assessment
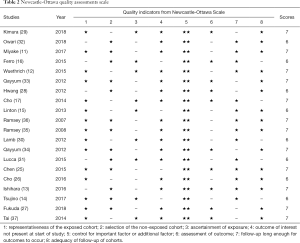
Two independent researchers (F Qi and Y Xu) were assigned for the whole selection process and discussion with a third investigator (Y Zheng) if disagreements exist. We extracted the following data according to prepared standard form: first author, publication year, cancer type, area, treatment, study design, sample size (total patients), endpoints (corresponding HRs with 95% CIs), follow-up and definition of GPS/mGPS. Kaplan-Meier curves were used to extract HR with 95% CI (20,21) if it could not be obtained directly from the article. Quality of each enrolled study was evaluated by the Newcastle-Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm), which was a useful tool for the quality assessment of non-randomized studies (22).
Statistical analysis
The whole analysis process was performed by Stata software (version 12.0; StataCorp LP, College Station, TX, USA). Pooled HRs were calculated by HRs with 95% CIs from each study. Moreover, heterogeneity was evaluated according to Higgins I2 and Cochran’s Q test. The random effect model (DerSimonian-Laird method) (23) was applied if significant heterogeneity existed (P<0.10 or I2>50%). Otherwise, the fixed effect model (Mantel-Haenszel method) (24) would be utilized. Publication bias was assessed using Egger’s linear regression test and Begg’s funnel plot, and P<0.05 was thought to have statistical significance. Additionally, sensitivity analyses were conducted to check the reliability and stability of the pooled results by excluding each study once a time consecutively.
Results
Study characteristics
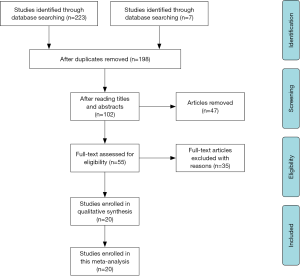
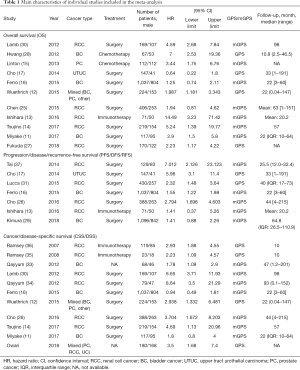
After careful selection, a total of 20 studies (11-17,25-37) were eventually enrolled in this meta-analysis, and detailed information of selection process was shown in Figure 1. Baseline characteristics of eligible researches were present in Table 1 and the NOS scores of included studies were all above 6 (detailed rankings were in Table 2). Generally, different studies focused on different urological cancers [1 study on PC, 5 studies on BC, 11 studies on RCC, 1 study on upper tract urothelial carcinoma (UTUC) and 2 studies on mixed cancer types]. In terms of treatment methods, immunotherapy was studied in 3 articles, surgery was studied in 13 articles, chemotherapy was in studied 2 articles and treatment method was not available in 2 articles. Overall, 11 articles explored the role of GPS/mGPS for OS, 10 for CSS, 1 for DFS, 1 for PFS, 5 for RFS and 1 for DSS.
Full table
Full table
GPS/mGPS and OS in urological cancers
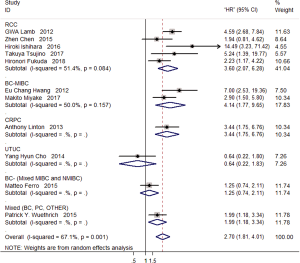
Eleven studies discussed the prognostic role of GPS/mGPS in urological cancers on OS. The results showed that relatively elevated pre-treatment GPS/mGPS was related to worse OS outcomes (pooled HR =2.70; 95% CI, 1.81–4.01) (Figure 2A). Subgroup analyses by cancer type for OS indicated that high level of pre-treatment GPS/mGPS yielded a worse OS in BC and RCC, (pooled HR =2.71; 95% CI, 1.08–6.82; pooled HR =3.60; 95% CI, 2.07–6.28, respectively) (Figure 2B), the same in MIBC after further classification (pooled HR =4.14; 95% CI, 1.77–9.65) (Figure S1). In terms of treatment methods, pre-treatment GPS/mGPS could be a negative predictor for OS (Surgery: pooled HR =2.16; 95% CI, 1.43–3.26; Chemotherapy: pooled HR =4.41; 95% CI, 2.27–8.58, separately) (Figure 2C). Lastly, both mGPS and GPS played a predictive role for OS (GPS: pooled HR =2.12; 95% CI, 1.04–4.32; mGPS: pooled HR =3.12; 95% CI, 1.87–5.20) (Figure 2D).

GPS/mGPS and CSS/DSS in urological cancers
A total of 11 studies investigated the role of GPS/mGPS on CSS/DSS. Conclusions could be drawn that relatively higher pre-treatment GPS/mGPS was associated with worse CSS/DSS (pooled HR =2.90; 95% CI, 2.00–4.22) (Figure 3A). Subsequent stratified analysis by cancer type for CSS/DSS proved that high level of pre-treatment GPS/mGPS led to worse CSS/DSS in RCC (pooled HR =4.12; 95% CI, 2.69–6.30), while no predictive significance in BC (pooled HR =1.46; 95% CI, 0.97–2.22) (Figure 3B). As for treatment methods, pre-treatment GPS/mGPS could be a negative predictor for CSS/DSS (Surgery: pooled HR =3.28; 95% CI, 1.73–6.20; Immunotherapy: pooled HR =2.72; 95% CI, 1.87–3.96, separately) (Figure 3C). Obviously, both mGPS and GPS were prognostic factors for CSS/DSS (GPS: pooled HR =2.87; 95% CI, 2.11–3.91; mGPS: pooled HR =3.00; 95% CI, 1.60–5.63) (Figure 3D).

GPS/mGPS and DFS/PFS/RFS in urological cancers
Seven studies explored the prognostic role of GPS/mGPS on DFS/PFS/RFS in this study. Results reveled that high levels of pre-treatment GPS/mGPS could result in worse DFS/PFS/RFS outcomes (pooled HR =2.43; 95% CI, 1.62–3.66) (Figure 4A). Subgroup analyses by cancer type for DFS/PFS/RFS demonstrated that pre-treatment GPS/mGPS was negatively correlated with DFS/PFS/RFS outcomes in BC and RCC (pooled HR =1.52; 95% CI, 1.23–1.88; pooled HR =2.66; 95% CI, 1.82–3.90; respectively) (Figure 4B). When it came to treatment methods, pre-treatment GPS/mGPS could be a negative predictor for DFS/PFS/RFS (Surgery: pooled HR =2.54; 95% CI, 1.65–3.92) (Figure 4C). Finally, both mGPS and GPS were all important prognostic factors for DFS/PFS/RFS (GPS: pooled HR =3.61; 95% CI, 1.43–9.07; mGPS: pooled HR =1.99; 95% CI, 1.32–2.99) (Figure 4D).

Sensitivity analysis
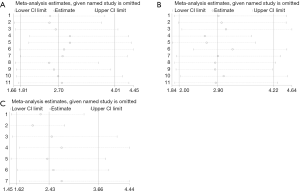
In order to discover the individual influence on the whole, we performed the sensitivity analysis by calculating the remained part by omitting one single study each time. Obviously, our results indicated that no single study could influenced the pooled HRs with 95% CIs significantly, indicating that our results were reliable (Figure 5).
Publication bias
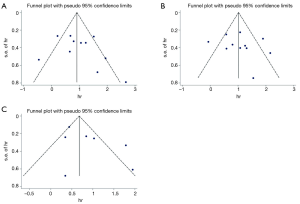
As shown in Figure 6, publication bias was evaluated based on the Egger’s linear regression test Begg’s funnel plot. All P values for OS or CSS/DSS or DFS/RFS/PFS were more than 0.05 (PEgger: OS: 0.329; CDD/DSS: 0.501; DFS/RFS/PFS:0.147), which meant that no significant bias existed.
Discussion
Previous studies (38-40) has already recognized that host inflammatory response was a vital determinant of disease progression. Elevated CRP, an evidence of SIR, had been identified as a negative prognostic factor in many cancers, such as thymic epithelial tumors (41), lung cancer (42), gastric cancer (43), PC and so on. Thurner et al. (44) identified that elevated plasma CRP (≥8.6 mg/L) was a strong prognostic predictor for poor survival in patients with PC, which was independent of other factors such as Gleason score, tumor stage and prostate specific antigen when diagnosed. Also, preoperative serum albumin level has already been recognized as a valuable factor on prognosis prediction in patients with various cancer types. Ayhan et al. (45) reported that preoperative albumin level was an independent predictive factor for OS in debulked epithelial ovarian cancer patients. Lambert et al. (46) found that pre-treatment albumin levels had a bearing on higher mortality in BC. A research conducted by Heys et al. (47) demonstrated that the presence of lower pre-treatment albumin level was tightly related to poorer survival in patients with colorectal cancer.
GPS, a combination of serum and CRP, was first introduced by Forrest (48,49) to establish a new predictive system for inoperable non-small cell lung cancer patients. Forrest discovered that GPS was an independent predictor of OS (HR 1.88; 95% CI, 1.25–2.84, P=0.002), which could provide additional prognostic information for clinical practice. Ramsey et al. (36) reported that in metastatic RCC patients, GPS could predict survival outcomes independent of former scoring systems. In 2007, McMillan et al. (50) first suggested to modify GPS into mGPS evaluating the prognostic effect of SIR on patients who underwent resection for rectal and colon cancers, and he believed that hypoalbuminemia was dependent on the presence of SIR in colorectal patients. Additionally, the prognostic role of mGPS had been proved in various cancer types, such as colorectal cancer (51), esophageal cancer (52), lung cancer (53) and so on. Fan et al. (54) compared GPS and mGPS in prognosis evaluation, and he put forward that GPS was superior to mGPS in non-small cell lung cancer patients. However, further studies are needed to discuss the comparison between GPS and mGPS. Furthermore, the concepts of sensitive mGPS (55) (S-mGPS) and high-sensitivity modified Glasgow prognostic score (HS-mGPS) (56,57) were proposed, and their prognostic effect had been proved.
In our research, 20 studies were enrolled to explore the relationship between GPS/mGPS and survival outcomes. The pooled results showed that lower pre-treatment GPS/mGPS level was closely related to better survival outcomes including OS, CSS/DSS and DFS/PFS/RFS. Obviously, both GPS and mGPS played an important in prognosis prediction. Furthermore, subgroup analysis by treatment type, GPS/mGPS and cancer type acquired the same conclusions. In sum, a low level of pre-treatment GPS/mGPS may indicate better survival outcomes. Only one article (17) was on the relationship between OS and GPS/mGPS in UTUC, and in that research Cho et al. suggested that GPS had no OS prediction significance (HR =0.64; 95% CI, 0.22–1.80) which was not consistent with the pooled result. We thought it was because of the relatively fewer articles in the group and uncontrollable bias. Study (not included in this meta-analysis because of the exclusion criteria and low evidence level) conducted by Suyama (58) investigated the prognostic significance of GPS in upper urinary urothelial carcinoma provided useful information, he claimed that GPS was an independent factor which could predict overall death in patients with urothelial carcinoma significantly (HR =6.18; 95% CI, 1.85–20.60).
This study was the first attempt for us to investigate the prognostic value of pre-treatment GPS/mGPS in urological cancers. Additionally, the strict inclusion criteria for enrolled articles made it more convincing and robust. Nevertheless, some potential limitations should not be ignored. Firstly, unavoidable bias may exist because most of the included studies were retrospective researches rather than prospective articles. Secondly, the entire heterogeneity was huge in some analysis processes, but the heterogeneity diminished when in subgroup analysis. Thirdly, relevant studies were too few in some subgroup analysis to obtain a convincing which made uncontrollable bias may exist (Cancer type: there was only one article on PC, and only one article on UTUC. Treatment type: only two articles on chemotherapy and only three articles on immunotherapy). Last but not least, further prospective randomized controlled trials were needed and upcoming studies should solve above difficulties before widely clinical application.
Conclusions
Our study shed light on that GPS/mGPS might be an independent prognostic factor in urological cancers, indicating that a lower level of pre-treatment GPS/mGPS was closely related to better survival outcomes (OS, DFS/RFS/PFS, CSS/DSS). However, deep exploration was restricted because of the limited researches. Therefore, higher quality randomized controlled trials and large sample size studies are still needed to further verify our results.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81702520), Medical Research Project of Jiangsu Provincial Health and Family Planning Commission (No. H2018052), Research Project of Jiangsu Cancer Hospital (No. ZN201602), and the young talents program of Jiangsu Cancer Hospital (No. 2017YQL-04).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Braun DA, Burke KP, Van Allen EM. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin Cancer Res 2016;22:5642-50. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005;353:2477-90. [Crossref] [PubMed]
- Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer 2018;18:285. [Crossref] [PubMed]
- Qi F, Zhou X, Wang Y, et al. Pre-treatment prognostic nutritional index may serve as a potential biomarker in urinary cancers: a systematic review and meta-analysis. Cancer Cell Int 2018;18:207. [Crossref] [PubMed]
- Karl A, Rittler P, Buchner A, et al. Prospective assessment of malnutrition in urologic patients. Urology 2009;73:1072-6. [Crossref] [PubMed]
- Nozoe T, Matono R, Ijichi H, et al. Glasgow Prognostic Score (GPS) can be a useful indicator to determine prognosis of patients with colorectal carcinoma. Int Surg 2014;99:512-7. [Crossref] [PubMed]
- Zhu J, Wang H, Liu CC, et al. The Glasgow Prognostic Score (GPS) is a novel prognostic indicator in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer Res Clin Oncol 2016;142:2339-45. [Crossref] [PubMed]
- Omichi C, Nakamura K, Haraga J, et al. Glasgow prognostic score is an independent marker for poor prognosis with all cases of epithelial ovarian cancer. Cancer Med 2016;5:1074-80. [Crossref] [PubMed]
- Miyake M, Morizawa Y, Hori S, et al. Integrative Assessment of Pretreatment Inflammation-, Nutrition-, and Muscle-Based Prognostic Markers in Patients with Muscle-Invasive Bladder Cancer Undergoing Radical Cystectomy. Oncology 2017;93:259-69. [Crossref] [PubMed]
- Wuethrich PY, Vidal A, Burkhard FC. There is a place for radical cystectomy and urinary diversion, including orthotopic bladder substitution, in patients aged 75 and older: Results of a retrospective observational analysis from a high-volume center. Urol Oncol 2016;34:58. e19-27.
- Ishihara H, Kondo T, Omae K, et al. Sarcopenia and the Modified Glasgow Prognostic Score are Significant Predictors of Survival Among Patients with Metastatic Renal Cell Carcinoma Who are Receiving First-Line Sunitinib Treatment. Target Oncol 2016;11:605-17. [Crossref] [PubMed]
- Tsujino T, Komura K, Matsunaga T, et al. Preoperative Measurement of the Modified Glasgow Prognostic Score Predicts Patient Survival in Non-Metastatic Renal Cell Carcinoma Prior to Nephrectomy. Ann Surg Oncol 2017;24:2787-93. [Crossref] [PubMed]
- Linton A, Pond G, Clarke S, et al. Glasgow prognostic score as a prognostic factor in metastatic castration-resistant prostate cancer treated with docetaxel-based chemotherapy. Clin Genitourin Cancer 2013;11:423-30. [Crossref] [PubMed]
- Ferro M, De Cobelli O, Buonerba C, et al. Modified Glasgow Prognostic Score is Associated With Risk of Recurrence in Bladder Cancer Patients After Radical Cystectomy: A Multicenter Experience. Medicine (Baltimore) 2015;94:e1861. [Crossref] [PubMed]
- Cho YH, Seo YH, Chung SJ, et al. Predictors of intravesical recurrence after radical nephroureterectomy for upper urinary tract urothelial carcinoma: an inflammation-based prognostic score. Korean J Urol 2014;55:453-9. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Crumley AB, McMillan DC, McKernan M, et al. Evaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancer. Br J Cancer 2006;94:637-41. [Crossref] [PubMed]
- Williamson PR, Smith CT, Hutton JL, et al. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- Chen Z, Shao Y, Fan M, et al. Prognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol 2015;8:14893-900. [PubMed]
- Cho DS, Kim SI, Choo SH, et al. Prognostic significance of modified Glasgow Prognostic Score in patients with non-metastatic clear cell renal cell carcinoma. Scand J Urol 2016;50:186-91. [Crossref] [PubMed]
- Fukuda H, Takagi T, Kondo T, et al. Prognostic value of the Glasgow Prognostic Score for patients with metastatic renal cell carcinoma treated by cytoreductive nephrectomy. Int J Clin Oncol 2018;23:539-46. [Crossref] [PubMed]
- Hwang EC, Hwang IS, Yu HS, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol 2012;42:955-60. [Crossref] [PubMed]
- Kimura S, D'Andrea D, Soria F, et al. Prognostic value of modified Glasgow Prognostic Score in non-muscle-invasive bladder cancer. Urol Oncol 2019;37:179 e19-28.
- Lamb GW, Aitchison M, Ramsey S, et al. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: basis of new prognostic scoring systems. Br J Cancer 2012;106:279-83. [Crossref] [PubMed]
- Lucca I, de Martino M, Hofbauer SL, et al. Comparison of the prognostic value of pretreatment measurements of systemic inflammatory response in patients undergoing curative resection of clear cell renal cell carcinoma. World J Urol 2015;33:2045-52. [Crossref] [PubMed]
- Owari T, Miyake M, Nakai Y, et al. A Genitourinary Cancer-specific Scoring System for the Prediction of Survival in Patients with Bone Metastasis: A Retrospective Analysis of Prostate Cancer, Renal Cell Carcinoma, and Urothelial Carcinoma. Anticancer Res 2018;38:3097-103. [PubMed]
- Qayyum T, McArdle P, Hilmy M, et al. A prospective study of the role of inflammation in bladder cancer. Curr Urol 2013;6:189-93. [Crossref] [PubMed]
- Qayyum T, McArdle PA, Lamb GW, et al. Prospective study of the role of inflammation in renal cancer. Urol Int 2012;88:277-81. [Crossref] [PubMed]
- Ramsey S, Aitchison M, Graham J, et al. The longitudinal relationship between the systemic inflammatory response, circulating T-lymphocytes, interleukin-6 and -10 in patients undergoing immunotherapy for metastatic renal cancer. BJU Int 2008;102:125-9. [Crossref] [PubMed]
- Ramsey S, Lamb GW, Aitchison M, et al. Evaluation of an inflammation-based prognostic score in patients with metastatic renal cancer. Cancer 2007;109:205-12. [Crossref] [PubMed]
- Tai CG, Johnson TV, Abbasi A, et al. External validation of the modified Glasgow prognostic score for renal cancer. Indian J Urol 2014;30:33-7. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-63. [Crossref] [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. [Crossref] [PubMed]
- Nakagawa K, Sho M, Akahori T, et al. Significance of the inflammation-based prognostic score in recurrent pancreatic cancer. Pancreatology 2019;19:722-8. [Crossref] [PubMed]
- Janik S, Bekos C, Hacker P, et al. Elevated CRP levels predict poor outcome and tumor recurrence in patients with thymic epithelial tumors: A pro- and retrospective analysis. Oncotarget 2017;8:47090-102. [Crossref] [PubMed]
- Shao N, Cai Q. High pretreatment serum C-reactive protein level predicts a poor prognosis for combined small-cell lung cancer. Tumour Biol 2015;36:8465-70. [Crossref] [PubMed]
- Kong F, Gao F, Chen J, et al. Elevated serum C-reactive protein level predicts a poor prognosis for recurrent gastric cancer. Oncotarget 2016;7:55765-70. [Crossref] [PubMed]
- Thurner EM, Krenn-Pilko S, Langsenlehner U, et al. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer 2015;51:610-9. [Crossref] [PubMed]
- Ayhan A, Gunakan E, Alyazici I, et al. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet 2017;296:989-95. [Crossref] [PubMed]
- Lambert JW, Ingham M, Gibbs BB, et al. Using preoperative albumin levels as a surrogate marker for outcomes after radical cystectomy for bladder cancer. Urology 2013;81:587-92. [Crossref] [PubMed]
- Heys SD, Walker LG, Deehan DJ, et al. Serum albumin: a prognostic indicator in patients with colorectal cancer. J R Coll Surg Edinb 1998;43:163-8. [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 2004;90:1704-6. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028-30. [Crossref] [PubMed]
- McMillan DC, Crozier JE, Canna K, et al. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 2007;22:881-6. [Crossref] [PubMed]
- Kishiki T, Masaki T, Matsuoka H, et al. Modified Glasgow prognostic score in patients with incurable stage IV colorectal cancer. Am J Surg 2013;206:234-40. [Crossref] [PubMed]
- Jomrich G, Hollenstein M, John M, et al. The modified glasgow prognostic score is an independent prognostic indicator in neoadjuvantly treated adenocarcinoma of the esophagogastric junction. Oncotarget 2018;9:6968-76. [Crossref] [PubMed]
- Lv Y, Pan Y, Dong C, et al. Modified Glasgow Prognostic Score at Recurrence Predicts Poor Survival in Resected Non-Small Cell Lung Cancer (NSCLC) Patients. Med Sci Monit 2017;23:3780-8. [Crossref] [PubMed]
- Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol 2016;142:1285-97. [Crossref] [PubMed]
- Tian R, Zhang F, Sun P, et al. The preoperative sensitive-modified Glasgow prognostic score is superior to the modified Glasgow prognostic score in predicting long-term survival for esophageal squamous cell carcinoma. Oncotarget 2016;7:67485-94. [Crossref] [PubMed]
- Takeno S, Hashimoto T, Shibata R, et al. The high-sensitivity modified Glasgow prognostic score is superior to the modified Glasgow prognostic score as a prognostic predictor in patients with resectable gastric cancer. Oncology 2014;87:205-14. [Crossref] [PubMed]
- Nakamura T, Matsumine A, Asanuma K, et al. The value of the high-sensitivity modified Glasgow prognostic score in predicting the survival of patients with a soft-tissue sarcoma. Bone Joint J 2015;97-B:847-52. [Crossref] [PubMed]
- Suyama T, Kanbe S, Maegawa M, et al. Prognostic significance of inflammation-based prognostic scoring in patients with upper urinary tract urothelial carcinoma. Int Braz J Urol 2019;45:541-8. [Crossref] [PubMed]