A modified pleurodesis in treating postoperative chylothorax
Introduction
Chylothorax, a postoperative complication of thoracic surgery, is defined as the accumulation of lymphatic fluid (chyle) in the pleural space caused by injuries or obstruction of the thoracic duct, the right lymphatic duct, or their branches. Chyle is rich in electrolytes and lipids (1), and the volume of chyle produced depends mostly on the amount and types of foods ingested particularly fatty acids. The severity of this disease depends on the volume of chyle leakage; severe chylothorax can lead to nutritional disorders, immunological dysfunction, and respiratory distress.
Chylothorax has been reported to occur in 0.5–2.0% of thoracic surgery patients (2,3), and its incidence may increase as surgical oncology makes greater use of lymphadenectomy. Chylothorax may be particularly frequent after esophageal surgery because the esophagus and thoracic duct are close to each other.
For traditionally postoperative chylothorax, conservative, non-surgical approaches such as thorough drainage, maintenance of electrolyte equilibrium, and essential parenteral nutrition or a low-fat diet to limit chyle generation, have been the preferred treatment types (4,5). These conservative approaches tend to cure more than 50% of patients, and the remainder needs to undergo a second surgery to repair the lymphatic leak or ligate the thoracic duct. However, the second surgery has a success rate of 90–95% (6,7). Approximately 5% of patients do not respond satisfactorily to these surgical or conservative approaches. These patients with so-called refractory chylothorax are at a high risk of poor prognosis. Chemical pleurodesis, in which chemicals like talc slurry, bleomycin, and hypertonic glucose are injected into the pleural space to promote pleurodesis, has long been described as an effective technique to treat chylothorax (1,8,9). However, according to our observation, if we consider volume of pleural drainage less than 200 mL/d as the criteria of chest tube removal, there will be great variation in the required time for treatment. And, in some cases, the period will be so long that massive lymphatic fluid loss will lead to severe malnutrition and an even worse situation.
We chanced upon a special case of chylothorax in a patient whose condition did not improve after conservative treatment or a second surgery to ligate the thoracic duct. During the third surgery, we observed extensive adhesions between the lung and chest wall, which led us to wonder whether simply clamping or removing the chest tube would be better than a third surgery. As adhesions formed once we stopped drainage, there was no potential space for chyle collection. Thus, we inferred that chyle effusion would stop. Without persistent chyle overflow, the lymphatic leak should repair itself and allow for the chylothorax to resolve. We have successfully tested this hypothesis in some refractory chylothorax patients at West China Hospital and four of its affiliated hospitals since 2010, and we wished to examine rigorously its safety and efficacy in order to determine its usefulness as a treatment guide for modified pleurodesis.
Methods
Ethical review
The study was approved by the Clinical Trials and Biomedical Ethics Committee of the hospital (No. 2015-112). The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the institutional review board of West China Hospital and its affiliated regional hospitals, and written informed consent was obtained from every participant. All necessary materials were analyzed anonymously. No financial support was received for this study.
Patients
Patients who underwent thoracic surgery for any reason at West China Hospital and 5 of its affiliated regional hospitals (Shang Jing Nan Fu Hospital of Chengdu, The Second People’s Hospital of Sichuan Province, The Third People’s Hospital of Zigong, the No. 903 Military Hospital of Jiangyou, The Gansu Provincial Hospital) between July 2010 and December 2015 and who subsequently developed chylothorax that did not resolve after conservative treatment, pleurodesis (one or several injections of a solution containing 100 mL 50% glucose and 20 mL 1% lidocaine into the chest tube), or surgical treatment, were consecutively enrolled in the study.
Diagnosis of postoperative chylothorax
Postoperative chylothorax was diagnosed based on the following criteria: (I) no manifestation of preoperative pleural effusion based on routine examinations such as contrast-enhanced computed tomography (CT); (II) excessive postoperative pleural effusion; (III) appearance of milky, white, yellow, serous or blood-stained fluid in the chest tube; (IV) pleural fluid triglyceride levels >1.24 mmol/L (110 mg/dL) combined with cholesterol levels <5.18 mmol/L (200 mg/dL) or the presence of chylomicrons in the pleural fluid (1,10).
Modified pleurodesis regimen
After enrollment, patients were provided with completed pleural drainage, partial parenteral nutrition, and a low-fat diet. Pleurodesis was promoted by a daily injection of a solution containing 100 mL 50% glucose and 20 mL 1% lidocaine into the chest tube; for diabetic patients, interleukin-2 (IL-2) (100 mL normal saline and one million IU) was used to replace 50% Glucose. After each injection, the chest tube was clamped for 1 h. During this hour, the patient was asked to engage in light exercise (e.g., gently rolling in bed) so that the solution could diffuse throughout the pleural space, and then the chest tube was released. This chemical pleurodesis lasted for 7 days. Drainage volume was recorded on a daily basis during the pleurodesis.
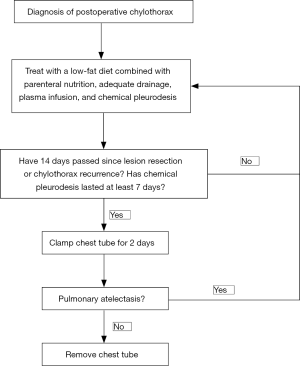
Usually, on the 7th day after enrollment, regardless of the volume of pleural drainage, the chest tube was clamped for 2 days. If the chest X-ray showed no presence of severe pulmonary atelectasis within 2 days (i.e., tight pleural adhesions formed), the chest tube was removed. If the patient showed partial pulmonary atelectasis, we also removed the tube, with the respiratory function of each patient closely monitored by chest X-ray until the pleural fluid was slowly absorbed. Participants were administered 400–600 mL fresh frozen plasma intravenously every 2–4 days to compensate for massive chyle loss, and during this entire period, conservative measures including partial parenteral nutrition and low-fat diets continued. All patients were followed up with for more than 3 months (Figure 1).
Statistical analysis
Differences in daily drained volume before and after chemical pleurodesis were assessed for significance using the paired-samples t-test. Data were statistically analyzed using SPSS22.0 (IBM, Chicago, IL, USA). A two-sided P value less than 0.05 was considered statistically significant.
Results
During the recruitment period, a total of 45 patients diagnosed as refractory chylothorax were enrolled in our study. Before enrollment, they received conservative therapies (fasting, parenteral nutrition, and adequate drainage) or pleurodesis (single or several injections of hypertonic glucose) or second surgery for thoracic duct ligation, but showed unsatisfactory response to these treatments. After 5–14 days of such therapies, pleural drainage still exceeded 900 mL per day. When the treatment progressed, 11 patients were excluded because the drainage decreased significantly during the course of pleurodesis and clamping the tube was no longer necessary.
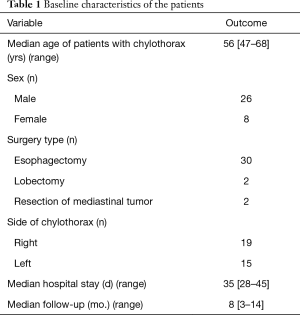
There were 34 remaining patients aged between 47 and 68 years old, 26 of who were male and 8 of whom were female; 2 had undergone right upper lobectomy, 2 had undergone resection of mediastinal tumor, and the remainder had undergone esophagectomy (Table 1). In all patients, chylothorax developed on the same side as the initial thoracic surgery (right side, n=19; left side, n=15). Thoracic duct ligation was performed in 14 patients during the initial thoracic surgery and in another 8 during a second surgery.
Full table
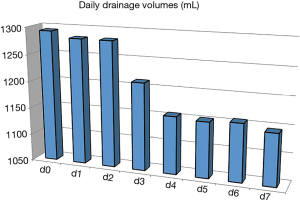
Figure 2 shows the change of pleural drainage volume before, during, and after the repeated chemical pleurodesis. A slight decrease of drainage was observed after the 3rd day of pleurodesis (P<0.05), but drainage was still excessive at the end of treatment.
Over the course of the repeated pleurodesis promotion and during follow-up of the 34 patients, we observed 3 types of results.
Type 1
In 10 patients, the pleural fluid did not increase by chest X-ray on day 2 after chest tube clamping; the tubes were removed, and the patients were discharged.
Type 2
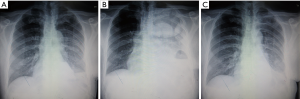
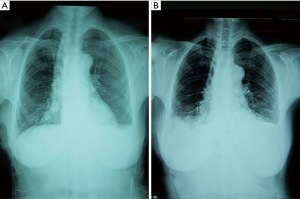
Three patients felt shortness of breath and tightness of the chest after tube clamp. Chest X-ray indicated multiple encapsulated effusions on the affected side. We also removed the tubes. Symptomatic treatments like oxygen inhalation were then administered to these patients. Finally, symptoms and pleural fluid gradually disappeared within 2 weeks (Figure 3).
Type 3
This was the most common result in our study. In the other 21 patients, a blunting costophrenic angle was observed by chest X-ray on day 2 after the chest tube was clamped (Figure 4). Usually, after we removed the tubes, the effusion was gradually absorbed several days later. However, 1 of these 21 patients was discharged on day 16 after the initial surgery and was readmitted for severe dyspnea 2 weeks later. Chest X-ray showed massive pleural effusion and complete atelectasis of the left lung, which indicated a recurrent chylothorax. Closed thoracic drainage was immediately performed, and the procedures mentioned in the methods section were applied again. On this occasion, this patient received a type 2 result. The patient was discharged on day 21 after readmission (Figure 5). Although chylothorax relapsed, this patient was still cured with this modified pleurodesis.
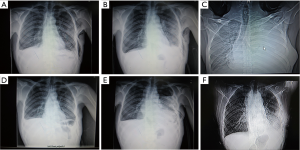
Apart from the single patient mentioned above, none of the patients showed recurrence during the follow-up of 3 months from the date of the final discharge. As of this writing and following the second round of modified pleurodesis, the patient with relapsed chylothorax has not recurred again.
Several adverse events were observed in the 34 patients. Electrolyte imbalance due to persistent chyle loss is reported to be very common (6) and occurred in these patients, as did chronic weakness despite normal nutritional indexes (debility of unknown origin). The first 2 patients recruited into the study, who did not receive fresh frozen plasma but intravenous human serum albumin and parenteral nutrition instead, showed debility. One of them also developed a pulmonary infection, and he improved after receiving intravenous plasma infusion and anti-infective therapy. None of the other patients showed debility after receiving freshly thawed frozen plasma. Six patients developed electrolyte imbalance. Five patients suffered allergic reactions during the plasma infusion; these patients were treated successfully using anti-allergic treatments. No pleural infection was observed in any of the participants.
Discussion
Traditionally, for patients with chylothorax, the chest tube is removed only when chyle drainage is no longer deemed excessive. Persistent chyle leakage often severely disturbs the internal environment or can even cause death. Thus, measures to stop the chyle leak as quickly as possible are considered essential for treating chylothorax.
Surgical repair of the lymphatic leak or ligation of the thoracic duct is the first choice if the chyle drainage does not stop after conservative treatment, with thoracic duct near-infrared (NIR) fluorescence imaging or magnetic resonance imaging being used to detect chylous fistulas in open surgical interventions of chylothorax (11-15). However, surgical treatment is not successful in every patient. Recently, several nonsurgical options to treat chylothorax have emerged, such as thoracic duct embolization and chemical pleurodesis (2). Embolization does not help patients who do not respond to major lymphatic duct ligation, and it requires special equipment and technical skill.
Chemical pleurodesis using various chemical agents offers a potentially more effective alternative (1,8,16,17), but this traditional pleurodesis has two shortcomings. The first relates to the agents being administrated. These agents are associated with adverse effects including allergic reactions, local cytotoxicity, and chest tube blockage (in the case of talc slurry), while other agents are even carcinogenic. These agents are typically administered only once in 20–40 mL water, which is too small a volume to cover the entire pleural space. Repeated injections of 100 mL 50% glucose solution can induce extensive adherence in the thoracic cavity and thereby safely and effectively treat pneumothorax and chylothorax (18,19). Over the last 10 years, we have cured many patients with prolonged postoperative air leakage by administering a daily intrapleural injection of 50% glucose. We normally mix the glucose with 1% lidocaine to reduce pleural irritation due to the hypertonic fluid. Additionally, for diabetic patients, interleukin-2 (IL-2) is used.
The second and more important shortcoming of traditional pleurodesis is the time needed for treatment. In some patients, a long wait is needed for the drainage drop. The long-term and massive lymphatic fluid loss will lead to severe malnutrition. In our study, 34 of the 45 patients did not have their drainage drop to an acceptable range on the 7th day of pleurodesis. We believe that if the pleurodesis had been continued, most of them would have been cured. However, the treatment time in these cases would have been much longer.
Traditionally, the chest tube is maintained in patients with chylothorax until the drainage tapers off and becomes non-chylous (20). This is to prevent continuous chyle effusion from causing pulmonary atelectasis and respiratory dysfunction after tube removal. As a result, chyle continues to leak, and since it cannot coagulate the way as blood does at a tear in the vasculature, the lymphatic crevasses are less likely to repair themselves. In our therapy, we enhance the pleurodesis and clamp/remove the chest tubes after tight pleural adhesions have formed, irrespective of daily drainage. The extensive pleural adhesions and strength of the bony chest wall prevent the lung from collapsing from the pleural effusion load, preserving respiratory function. Without continuous drainage and without potential space to collect chyle, the intrapleural pressure may soon increase to reach the pressure in the thoracic duct. Since there is no pressure gradient between the lumen of thoracic duct and the pleural space, the chyle leak will stop. Our results suggest that, as expected, this might facilitate the healing of lymphatic crevasses. In fact, Ragosta has reported a case of chylothorax being cured by increasing the mean airway pressure of mechanical ventilation (21), which is another example indicating an increase in intrapleural pressure might be useful to stop chyle leak.
In our approach, we clamped and removed the chest tube only after 7 days of chemical pleurodesis in 34 patients. Only 1 patient had chylothorax relapse with an adhesion not strong enough to withstand the pleural effusion load. Prolonging the time for chemical pleurodesis might prevent relapse, but will also increase the fluid loss. Large-scale trials should be carried out to determine the ideal time for chemical pleurodesis.
Common adverse effects in conservative therapy of chylothorax, such as electrolyte disturbance, malnutrition, and immunodeficiency, may also arise in the repeated pleurodesis procedures (1,2,5,12). Plasma infusion, together with enteral and parenteral nutritional support, seems to help prevent these adverse effects, while fresh frozen plasma serves to prevent debility, maintain immunological function, and accelerate recovery (22). Debility frequently appears if the patients do not receive plasma infusion, but how intravenous plasma administration relieves debility in our patients is unclear. Future studies should compare frozen plasma and serum albumin for their ability to prevent such weakness. Shortness of breath and chest tightness due to suppositional increase in intra-pleural pressure may also occur, though these can usually be relieved through symptomatic treatments.
Most clinicians recommend parenteral nutrition and/or a low-fat enteral diet to help alleviate chylothorax, although no definitive standards for nutrition therapy for chylothorax have been developed (22). Since our approach can take a relatively long time, patients typically lose large chyle volumes containing various nutrients and lymphocytes, so a single administration of parenteral nutrition may be insufficient for recovery (18,22). Therefore, our patients do not fast but instead receive a combination of parenteral nutrition with a low-fat diet rich in medium-chain triglycerides. The low-fat diet helps maintain the normal physiological function of the gastrointestinal tract without stimulating chyle production (4,23), making it compatible with our “time-consuming” therapy. In fact, one of our patients consumed several high-fat meals against protocol yet had a full recovery like the other patients.
Our experience suggests that successful application of modified pleurodesis depends on adhering to several rules. First, complete drainage is the prerequisite of the modified pleurodesis. The better the drainage is, the better the adhesion is. Chemical pleurodesis should not be initiated until a lack of pulmonary atelectasis has been confirmed. Additionally, since viscous chyle can obstruct the chest tube, smooth and effective drainage of the pleural effusion should be maintained before the chest tube is clamped. Second, chemical pleurodesis should continue for at least 7 days to ensure the formation of pleural adhesions strong enough to withstand the pleural effusion load. Third, fresh frozen plasma or frozen plasma (if patients do not have a coagulopathy problem) is important to keep the patients clear of electrolyte disturbance, immunodeficiency, and debility.
Some limitations of the study should be noted. While modified pleurodesis with an enhanced process of chemical pleurodesis and a shortened treatment time is effective and safe for patients with chylothorax, the requirement of large volumes of plasma exposes our patients to the same risks as any blood transfusion, including anaphylactic reaction, hemolysis, and transfusion-transmitted disease (24). Therefore, we recommend our procedures specifically only for treating refractory chylothorax rather than as a routine treatment for all patients with all chylothorax. Since there is no difference between traumatic and postoperative chylothorax in pathogenesis, we further speculate that this modified pleurodesis may be useful in post-traumatic cases. Large-scale randomized controlled trials or prospective cohort studies are needed to compare repeated pleurodesis with conventional treatments to verify the safety and efficacy for refractory chylothorax, as well as to explore the usefulness of this novel insight for other conditions and the potential factors influencing the recurrent accumulation of pleural effusions or pleural adhesion.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Clinical Trials and Biomedical Ethics Committee of the Hospital (No. 2015-112). The study protocol was approved by the institutional review board of West China Hospital and its affiliated regional hospitals, and written informed consent was obtained from every participant.
References
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med 2010;104:1-8. [Crossref] [PubMed]
- Chalret du Rieu M, Baulieux J, Rode A. Management of postoperative chylothorax. J Visc Surg 2011;148:e346-52. [Crossref] [PubMed]
- Bryant AS, Minnich DJ, Wei B, et al. The incidence and management of postoperative chylothorax after pulmonary resection and thoracic mediastinal lymph node dissection. Ann Thorac Surg 2014;98:232-5. [Crossref] [PubMed]
- Migliori C, Boroni G, Milianti S. Chylothorax. Minerva Pediatr 2010;62:89-91. [PubMed]
- Stager V, Le L, Wood RE. Postoperative chylothorax successfully treated using conservative strategies. Proc (Bayl Univ Med Cent) 2010;23:134-8. [Crossref] [PubMed]
- Zabeck H, Muley T, Dienemann H, et al. Management of chylothorax in adults: when is surgery indicated? Thorac Cardiovasc Surg 2011;59:243-6. [Crossref] [PubMed]
- Paul S, Altorki NK, Port JL, et al. Surgical management of chylothorax. Thorac Cardiovasc Surg 2009;57:226-8. [Crossref] [PubMed]
- Huang PM, Lee YC. A new technique of continuous pleural irrigation with minocycline administration for refractory chylothorax. Thorac Cardiovasc Surg 2011;59:436-8. [Crossref] [PubMed]
- Itkin M, Kucharczuk JC, Kwak A, et al. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg 2010;139:584-89. [Crossref] [PubMed]
- Skouras V, Kalomenidis I. Chylothorax: diagnostic approach. Curr Opin Pulm Med 2010;16:387-93. [Crossref] [PubMed]
- Cho HJ, Dong KK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: effective management and pleurodesis. Ann Thorac Surg 2014;97:408-13. [Crossref] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg 2007;32:362-9. [Crossref] [PubMed]
- Yu DX, Ma XX, Wang Q, et al. Morphological changes of the thoracic duct and accessory lymphatic channels in patients with chylothorax: detection with unenhanced magnetic resonance imaging. Eur Radiol 2013;23:702-11. [Crossref] [PubMed]
- Chang YC, Yen YT, Chang MC, et al. Localization of thoracic duct using heavily T2W MRI for intractable post-esophagectomy chylothorax-a case report. J Thorac Dis 2017;9:E109-14. [Crossref] [PubMed]
- Yang F, Zhou J, Li H, et al. Near-infrared fluorescence-guided thoracoscopic surgical intervention for postoperative chylothorax. Interact Cardiovasc Thorac Surg 2018;26:171-5. [Crossref] [PubMed]
- Jianjun Q, Song Z, Yin L, et al. Treatment of chylothorax with elemene. Thorac Cardiovasc Surg 2008;56:103-5. [Crossref] [PubMed]
- Rizzardi G, Loy M, Marulli G, et al. Persistent chylothorax in lymphangioleiomyomatosis treated by intrapleural instillation of povidone. Eur J Cardiothorac Surg 2008;34:214-5. [Crossref] [PubMed]
- Chen Y, Li C, Xu L, et al. Novel treatment for chylothorax after esophagectomy with 50% glucose pleurodesis. Ann Vasc Surg 2010;24:694.e9-13. [Crossref] [PubMed]
- Peng ZM, Wu ES. Clinical value of hypertonic glucose injection in the treatment of recurrent pneumothorax. Hunan Yi Ke Da Xue Xue Bao 2002;27:256-8. [PubMed]
- Cerfolio RJ. Chylothorax after esophagogastrectomy. Thorac Surg Clin 2006;16:49-52. [Crossref] [PubMed]
- Ragosta KG, Alfieris G. Chylothorax: a novel therapy. Crit Care Med 2000;28:1208-9. [Crossref] [PubMed]
- Smoke A, Delegge MH. Chyle leaks: consensus on management? Nutr Clin Pract 2008;23:529-32. [Crossref] [PubMed]
- de Beer HG, Mol MJ, Janssen JP. Chylothorax. Neth J Med 2000;56:25-31. [Crossref] [PubMed]
- Squires JE. Risks of transfusion. South Med J 2011;104:762-9. [Crossref] [PubMed]