Preemptive renal replacement therapy in post-cardiotomy cardiogenic shock patients: a historically controlled cohort study
Introduction
Post-cardiotomy cardiogenic shock (PCCS), which occurred in 2–6% of patients undergoing surgical revascularization or valvular surgery, is a severe type of complication after cardiac surgery with high mortality (1,2). Along with appreciation of pathophysiology of PCCS, various therapeutic strategies are emerging for clinical option, such as vasodilator, inotropic drugs and techniques of extracorporeal life support, etc. However, the outcome of PCCS is still poor. Optimization of therapeutic approaches to PCCS should be imperative.
Renal replacement therapy (RRT) is a common treatment to the PCCS patients with acute kidney injury (AKI). The effect of RRT timing on cardiac surgical patients has been reported in several studies (3,4), but the optimal timing of RRT initiation remains controversial (5). A meta-analysis of 15 studies has revealed that early RRT initiation decreases 28-day mortality, especially when it is started within 24 h after cardiac surgery in patients with AKI (6); However, the studies included adopted different definitions of the timing of early and late RRT precluding the establishment of definitive conclusions. Furthermore, the definitions of “early” and “late” RRT in the included studies were generally based on renal indicators such as urine output, blood urea nitrogen and serum creatinine (SCr) levels. The haemodynamic status of patients receiving RRT was not evaluated in most of these studies.
Recently, a system describing stages of cardiogenic shock (CS) from A to E (At risk, Beginning, Classic, Deteriorating and Extremis) was developed indicating that patients with CS often have dynamic clinical symptomatology and hemodynamics (7). Given the delicate nature of the kidney-heart interaction in cardiac surgical patients, RRT might provide an additional important platform for multiorgan support by potentially limiting the worsening of nonrenal organ dysfunction that may be exacerbated by AKI (8). Therefore, we supposed that preemptive RRT at early stages of PCCS with AKI might be benefit to outcomes of patients.
We conducted a historically controlled cohort study in PCCS patients with AKI based on different RRT initial strategies to elucidate whether the preemptive RRT strategy might improve outcomes.
Methods
This study was approved by the Ethical Committee of Zhongshan Hospital affiliated to Fudan University (No. B2016-147R) and all the patients have given their informed consent for participation in the current research study.
Patients
This is a historically controlled study on a cohort of patients who underwent cardiac surgery in Zhongshan Hospital which is affiliated with Fudan University, Shanghai, China. Zhongshan hospital is one of the largest centres for cardiovascular surgery in mainland China, that currently performs more than 4,000 adult cardiac surgery procedures per year. We collected data from patients who underwent cardiac surgery between September 2014 and November 2017. There were no changes in regards to cardiovascular surgeons and surgical techniques utilized within the study periods. Exclusion criteria were age under 18 years, survival for <48 h in the intensive care unit (ICU), severe cardiac surgery related complications, prior history of end-stage renal disease (ESRD) and underwent heart transplantations. PCCS was defined as follows (9): (I) persistent hypotension (mean artery pressure (MAP) <65 mmHg for more than 6 h) despite preload optimization; (II) use of high-dose vasoactive drugs [norepinephrine >0.4 µg/kg/min, epinephrine >0.2 µg/kg/min or epinephrine + (norepinephrine/2) >0.2 µg/kg/min]; (III) signs of impaired organ perfusion with at least one of the following criteria: (i) oliguria; (ii) increased serum lactate; (iii) cold, clammy skin; or (iv) altered mental status. AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 criteria as the absolute value of SCr increase ≥26.5 mmol/L within 48 h, an increase >50% compared to the baseline values within 7 days, or a urine output <0.5 mL/kg/h for ≥6 h. Complete renal recovery was defined as SCr level was no more than 0.5 mg/dL above the baseline value at discharge, whereas incomplete renal recovery was defined as failure to meet the criteria for complete renal recovery but without RRT treatment (10). No recovery was defined as SCr at discharge ≥ maximum SCr level during AKI episode, or a requirement for RRT. The percent fluid overload (PFO) = [total fluid input (L) − total fluid output (L)]/basal weight (kg) ×100% (11).
Indications for RRT
Before April 2016, RRT was often started depending on conventional indications or bedside decision-makings by attending doctors (intensivists and nephrologists) in patients with PCCS. The conventional indications included hyperkalemia >6.0 mmol/L, metabolic acidosis (pH <7.2), urine output <0.3 mL/kg/h for more than 24 h despite preload optimization, azotemia (SCr level >4 mg/dL), or evidence of fluid overload with pulmonary edema. As extension of our prior study (12), from May 2016, we adopted the preemptive RRT strategy for all PCCS patients with AKI in our cardiac surgical ICU. The preemptive RRT indications for patients with PCCS were defined as follows (12): (I) AKI in the absence of traditional indications for RRT; (II) persistent hypotension (MAP <65 mmHg for more than 6 h) with high-dose vasoactive drugs despite preload optimization; (III) low probability of rapid renal recovery according to the judgment of the intensivists and nephrologists. We used goal directed renal replacement therapy (GDRRT) for critical RRT patients since 2008, which was composed of almost all kinds of RRT modes and the dose, dialysate compositions, ultrafiltration rates, anticoagulation, duration of RRT sessions and RRT frequency were all adjusted dynamically according to the hemodynamic status. The goals were set up at RRT initiation including (I) solute control: (i) BUN ≤30 mmol/L, (ii) RRT dose 25–30 mL/kg/h; (II) volume control: (i) 24 h output ≥ input, (ii) reduction of peripheral edema; (III) metabolism control: (i) 3.5< K+ ≤5.5 mmol/L, (ii) 135< Na+ ≤145 mmol/L, (iii) pH ≥7.25, (iv) HCO3- ≥16 mmol/L, (v) lactic normal or near normal; (IV) hemodynamics: (i) MAP ≥65 mmHg, (ii) CVP 8–12 mmHg. There was no migration of RRT technological preferences during the whole study period.
Data collection
Two consecutive periods were analyzed: (I) Period A, when the indication of RRT was mainly based on conventional indicators (September 2014–April 2016); and (II) Period B, when all PCCS patients with AKI required RRT on the basis of preemptive indications (May 2016–November 2017). We collected data on demography characteristics, preoperative comorbidities, cardiac function and renal function. Perioperative data included cardiopulmonary bypass duration and aortic clamping duration. Postoperative hemodynamic variables included CVP, MAP, 24 h fluid input and output and vasoactive agent applications. The data was extracted and collected from both electronic medical system and nursing record sheets. The primary endpoints were in-hospital mortality, proportion of renal recovery and time from AKI to complete renal recovery. The secondary end points included length of hospital stay and ICU stay, duration of mechanical ventilation, RRT time and SCr at discharge.
Statistical analysis
Statistical analysis was conducted using SPSS Statistics for Windows (ver. 22, IBM Corp.). Normally distributed data are presented as the mean ± SD. Comparisons between groups were made using two independent sample t-tests. Medians (P25, P75) are used to present nonparametric data. A Wilcoxon test was used to assess two dependent variables, a nonparametric Mann-Whitney U test was used for independent variables, and a chi-square test was used for group comparisons. A P value <0.05 was assumed to represent a significant difference.
Results
Baseline characteristics
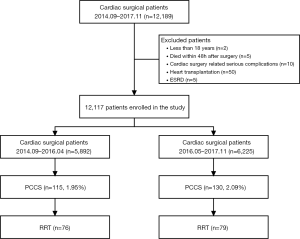
During the period from September 2014 to November 2017, a total of 12,189 patients underwent cardiac surgery in our center. Seventy-two patients were excluded because they were aged younger than 18 years (n=2), died within 48 h after surgery (n=5), severe cardiac surgery related complications (n=10), underwent heart transplantation (n=50) or ESRD (n=5). Therefore, 12,117 patients were enrolled in this study. The flow chart of the study was shown in Figure 1. There were 76 patients with PCCS who received RRT in Period A and 79 patients with PCCS who received RRT in Period B. There were no significant differences in sex, age, body mass index, preoperative comorbidities (hypertension and diabetes mellitus), New York Heart Association class >II, left ventricular ejection fraction, prior cardiac surgery, coronary angiography, duration between coronary angiography and surgery, baseline renal function as blood urea nitrogen, SCr, estimated glomerular filtration rate and mortality prediction score (EuroSCORE) between the groups. Intraoperative variables including emergency surgery, type of surgery, cardiopulmonary bypass duration, aortic clamp duration and ultrafiltration volume did not significantly differ between the two groups (Table 1). The Acute Physiology and Chronic Health Evaluation II score (APACHE II) was comparable between the two groups (25±7 vs. 24±7, P=0.244).
Full table
Comparison at initiation of RRT between the two groups
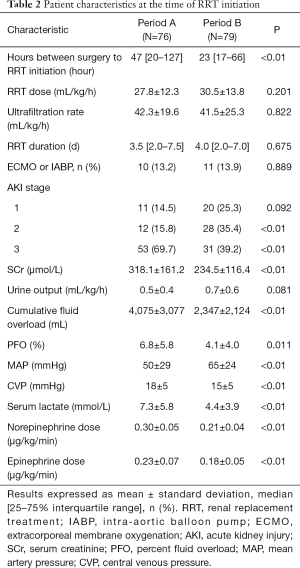
The duration from surgery to RRT initiation was significantly shorter in Period B than in Period A [23 (17, 66) vs. 47 (20, 127) h, P<0.01]. There was no significant difference in achieved RRT dose, ultrafiltration rate and RRT duration between the two groups. There were more patients with a lower AKI grade in Period B. The proportion of patients receiving MCS, including extracorporeal membrane oxygenation (ECMO) or an intra-aortic balloon pump (IABP) was comparable between both groups. The patients in Period B displayed a higher MAP, lower doses of vasoactive agents including norepinephrine and epinephrine, and lower level of CVP than those in Period A. The SCr level, cumulative fluid overload, PFO and lactic acid level were significantly lower in Period B (Table 2).
Full table
Changes within 72 h after RRT initiation
There was a trend of increasing MAP and decreasing CVP in Period B throughout the first 72 h after RRT initiation, whereas there was not much changes in Period A. The lactic acid level decreased significantly after RRT treatment in Period B, without changes in Period A. The doses of norepinephrine and epinephrine were also decreased very significantly after RRT initiation in Period B but not in Period A (Figure 2). The evolution of the hemodynamic parameters over time was different in survivors and non-survivors (Figures S1,S2).
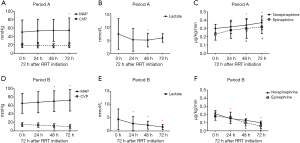
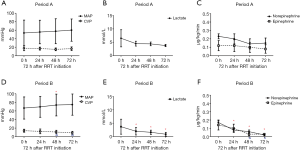
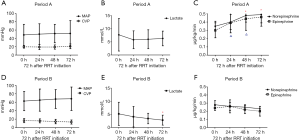
Primary and secondary outcomes
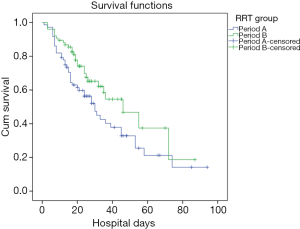
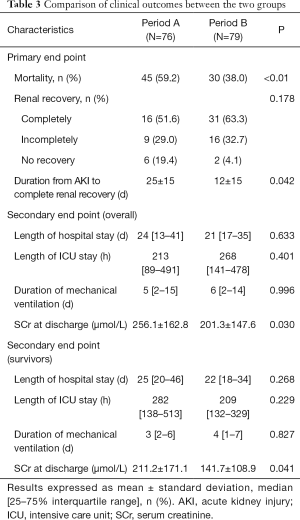
The hospital mortality was significantly lower in Period B than Period A (38.0% vs. 59.2%, P<0.01) (Figure 3). There were fewer patients with no renal recovery in Period B (4.1% vs. 19.4%). Patients in Period B displayed a significantly shorter time to completely renal recovery (12±15 vs. 25±15 d, P=0.042). Between the two groups, there were no significant differences in length of hospital stay [21 (17, 35) vs. 24 (13, 41) d, P=0.633], length of ICU stay [268 (141, 478) vs. 213 (89, 491) h, P=0.401] and duration of mechanical ventilation [6 (2, 14) vs. 5 (2, 15) d, P=0.996]. Patients in Period B exhibited significantly lower SCr levels at discharge than did those in Period A (201.3±147.6 vs. 256.1±162.8 µmol/L, P=0.030). In survivors, the length of hospital stay, length of ICU stay and duration of mechanical ventilation were comparable between two periods. SCr levels at discharge in Period B were significantly lower than in Period A (Table 3).
Full table
Discussion
CS is the most severe manifestation of acute heart failure which carries over 40% mortality in contemporary cohorts (13,14). Classically, the profile of “cold and wet” is the most frequent CS phenotype characterized by downstream hypoperfusion and upstream congestion (15), which might constitute the vicious cycle of circulatory failure. Inferentially, early correction of congestive state by RRT might ameliorate circulatory failure in CS patients. Successful management of CS patients is time dependent (16). The concept of “time to unload” accentuates the need to act quickly and earlier than is commonly practiced in the current setting.
In this preliminary study, preemptive initiation of RRT might played a role for early stabilizing hemodynamics. Subsequently, the strategy led to lower hospital mortality (38.0% vs. 59.2%) in patients with RRT. Preemptive RRT initiation was associated with lower mortality (23.1% vs. 39.1%) in those with PCCS as well. Additionally, it also accelerated the renal recovery after AKI. Although it is accepted that RRT should be initiated without delay in life threatening situations, the available data are insufficient to define the optimal timing of initiation of RRT beyond life-threatening situations (17-20).
Recently, three high-profile randomized controlled trials have been performed to determine the optimal timing of starting RRT in critically ill patients (21-23). The AKIKI trial and IDEAL-ICU study have demonstrated no significant difference with regard to mortality between an early and a delayed strategy for the initiation of RRT (21,23). In contrast, the ELAIN randomized clinical trial has revealed that early initiation of RRT improves patient survive as compared with that after delayed RRT (22). Notably, almost half (108/231, 46.8%) of the enrolled patients in the ELAIN study received cardiac surgery before admission to the ICU. It is worth mentioning that the definitions of “early” and “late” RRT in these 3 studies were principally based on renal indicators. We believe that the decision to initiate RRT should also take into consideration hemodynamic status and systemic condition in CS patients.
At present, there is still less powerful clinical demonstration of the optimal timing for RRT initiation for patients with CS, although two meta-analyses have revealed a potential benefit of early initiation of RRT in critically ill patients after cardiac surgery (6,24). In 2015, Combes and coworkers have found that, for patients with postcardiac surgery shock requiring high-dose of catecholamines, the early initiation of high-volume hemofiltration did not lower day-30 mortality and did not affect other important patient-centered outcomes (HEROICS study) (25). In the HEROICS study, almost 50% patients had received a mechanical assistance device (IABP or ECMO) which represented the most serious stage of CS. Of note, the main purpose of high-volume hemofiltration in the HEROICS study was to modulate the inflammatory milieu, not to provide renal support (26). However, results of the study did not support this indication. Accordingly, in our previous case mixed data, preemptive RRT were associated with lower hospital mortality, and faster and more frequent recovery of renal function (12). This finding encouraged us to initiate RRT actively in patients with PCCS (requiring high-dose catecholamines) in recent years. By contrast to the HEROICS study, we found that the in-hospital mortality in the preemptive RRT group was significantly lower. The preemptive RRT strategy also seemed to be beneficial in renal function recovery in these patients.
Another concern is that, the preemptive RRT strategy would inevitably expose some patients to “unnecessary” RRT, and these patients would have had more opportunity to recovery from AKI. In the AKIKI trial, delaying the initiation of RRT allowed almost half of the enrolled patients to recover from AKI without an RRT treatment course (21). In the current study, the frequency of RRT applications in PCCS patients in the two periods was 66.1% and 60.8%, respectively. Although the duration between the surgeries and RRT initiation was significantly shortened in the second period, the overuse of RRT did not seem to occur due to the preemptive strategy in this study.
However, we are aware that, our study had inevitable limitations. First, this was a single center and historical control study. For example, the retrospective data collection and outcome evaluation might have had certain biases. In addition, there were significantly differences regarding the patient characteristics at the time of RRT initiation between two groups. The condition prior to RRT in Period A was more severe than Period B, which might influence the interpretation of final results. However, given dynamic progression of CS, we supposed that relative less severe stage of CS might booster the effect of RRT. Third, as the clinicians were not blinded to the study, this may introduce a practice-related history bias in the treatment of the patients. Finally, in our study, we focused on patients with CS only, but the effects of timing of RRT in patients without CS were not under full evaluation as additional control.
Conclusions
Among PCCS patients, preemptive RRT compared with conventional initiation of RRT reduced mortality during the hospital stay. Preemptive RRT also led to faster and more frequent recovery of renal function. Further multicenter studies of this intervention are warranted. Our preliminary study supposed that preemptive initiation of RRT might be an effective approach to PCCS.
Acknowledgments
Funding: This article was supported by the Research Fund of Shanghai Municipal Health Commission (2019ZB0105) and grants from the Research Funds of Zhongshan Hospital (2019ZSYXQN34, 2019ZSQN13, 2018ZSQN53 and XYYX201922).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethical Committee of Zhongshan Hospital affiliated to Fudan University (No. B2016-147R), and all the patients have given their informed consent for participation in the current research study.
References
- Khorsandi M, Shaikhrezai K, Prasad S, et al. Advanced mechanical circulatory support for post-cardiotomy cardiogenic shock: a 20-year outcome analysis in a non-transplant unit. J Cardiothorac Surg 2016;11:29. [Crossref] [PubMed]
- Pérez Vela JL, Jimenez Rivera JJ, Alcala Llorente MA, et al. Low cardiac output syndrome in the postoperative period of cardiac surgery. Profile, differences in clinical course and prognosis. The ESBAGA study. Med Intensiva 2018;42:159-67. [PubMed]
- Yang XM, Tu GW, Zheng JL, et al. A comparison of early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials. BMC Nephrol 2017;18:264. [Crossref] [PubMed]
- Lai TS, Shiao CC, Wang JJ, et al. Earlier versus later initiation of renal replacement therapy among critically ill patients with acute kidney injury: a systematic review and meta-analysis of randomized controlled trials. Ann Intensive Care 2017;7:38. [Crossref] [PubMed]
- Zhang Z. No "optimal timing" of renal-replacement therapy in critically ill patients with acute kidney injury. Ann Transl Med 2018;6:S112. [Crossref] [PubMed]
- Zou H, Hong Q, Xu G. Early versus late initiation of renal replacement therapy impacts mortality in patients with acute kidney injury post cardiac surgery: a meta-analysis. Crit Care 2017;21:150. [Crossref] [PubMed]
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29-37. [PubMed]
- Bagshaw SM, Wald R. Strategies for the optimal timing to start renal replacement therapy in critically ill patients with acute kidney injury. Kidney Int 2017;91:1022-32. [Crossref] [PubMed]
- Levy B, Bastien O, Karim B, et al. Experts' recommendations for the management of adult patients with cardiogenic shock. Ann Intensive Care 2015;5:52. [PubMed]
- VA/NIH Acute Renal Failure Trial Network, Palevsky PM, Zhang JH, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7-20. [Crossref] [PubMed]
- Fülöp T, Pathak MB, Schmidt DW, et al. Volume-related weight gain and subsequent mortality in acute renal failure patients treated with continuous renal replacement therapy. ASAIO J 2010;56:333-7. [Crossref] [PubMed]
- Yang XM, Tu GW, Gao J, et al. A comparison of preemptive versus standard renal replacement therapy for acute kidney injury after cardiac surgery. J Surg Res 2016;204:205-12. [Crossref] [PubMed]
- Harjola VP, Lassus J, Sionis A, et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail 2015;17:501-9. [Crossref] [PubMed]
- Mebazaa A, Tolppanen H, Mueller C, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med 2016;42:147-63. [Crossref] [PubMed]
- van Diepen S, Katz JN, Albert NM, et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation 2017;136:e232-68. [Crossref] [PubMed]
- Tehrani B, Truesdell A, Singh R, et al. Implementation of a Cardiogenic Shock Team and Clinical Outcomes (INOVA-SHOCK Registry): Observational and Retrospective Study. JMIR Res Protoc 2018;7:e160. [Crossref] [PubMed]
- Vinsonneau C, Allain-Launay E, Blayau C, et al. Renal replacement therapy in adult and pediatric intensive care: Recommendations by an expert panel from the French Intensive Care Society (SRLF) with the French Society of Anesthesia Intensive Care (SFAR) French Group for Pediatric Intensive Care Emergencies (GFRUP) the French Dialysis Society (SFD). Ann Intensive Care 2015;5:58. [Crossref] [PubMed]
- Shiao CC, Huang TM, Spapen HD, et al. Optimal timing of renal replacement therapy initiation in acute kidney injury: the elephant felt by the blindmen? Crit Care 2017;21:146. [Crossref] [PubMed]
- Romagnoli S, Clark WR, Ricci Z, et al. Renal replacement therapy for AKI: When? How much? When to stop? Best Pract Res Clin Anaesthesiol 2017;31:371-85. [Crossref] [PubMed]
- Uhel F, Peters-Sengers H, van der Poll T. Initiation of renal replacement therapy in patients with sepsis: more to it than meets the eye. Ann Transl Med 2018;6:S130. [Crossref] [PubMed]
- Gaudry S, Hajage D, Schortgen F, et al. Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med 2016;375:122-33. [Crossref] [PubMed]
- Zarbock A, Kellum JA, Schmidt C, et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016;315:2190-9. [Crossref] [PubMed]
- Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of Renal-Replacement Therapy in Patients with Acute Kidney Injury and Sepsis. N Engl J Med 2018;379:1431-42. [Crossref] [PubMed]
- Liu Y, Davari-Farid S, Arora P, et al. Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2014;28:557-63. [Crossref] [PubMed]
- Combes A, Brechot N, Amour J, et al. Early High-Volume Hemofiltration versus Standard Care for Post-Cardiac Surgery Shock. The HEROICS Study. Am J Respir Crit Care Med 2015;192:1179-90. [Crossref] [PubMed]
- Palevsky PM. High-Volume Hemofiltration in Post-Cardiac Surgery Shock. A Heroic Therapy? Am J Respir Crit Care Med 2015;192:1143-4. [Crossref] [PubMed]