
Achyranthes bidentata polypeptide k suppresses neuroinflammation in BV2 microglia through Nrf2-dependent mechanism
Introduction
Microglia play an important role in immune defense and tissue repair of central nervous system (1). Under normal conditions, microglial cells are in a static state, participating in the maintenance of homeostasis in the brain. Factors such as brain damage, infection or harmful toxins activate microglia, causing neuroinflammation (2). Neuroinflammation can be good or bad for neurons in nearby areas, like a double-edged sword in the brain (3). The functional phenotypes of microglia largely determine the pros and cons of neuroinflammation, mainly the pro-inflammatory M1-type and the anti-inflammatory M2-type (4,5). M1-type microglia mediate inflammation by releasing pro-inflammatory mediators like tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, nitric oxide (NO), and reactive oxygen series (ROS), etc., while M2-type microglia inhibit inflammation mainly by producing anti-inflammatory factors, such as IL-4, IL-10, transformation growth factor β (TGF-β) (6-9). Some studies suggest that the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) is involved in the anti-inflammatory effect of the M2-type microglia (10,11). Nrf2 is a key factor of brain endogenous defense system, which can be produced by microglial cells in response to oxidative stress (12,13). Studies showed that activation of Nrf2 and its downstream heme oxygenase-1 (HO-1) could suppress lipopolysaccharide (LPS)-induced neuroinflammation both in vivo and in vitro (14-16). A large body of evidence demonstrate that nuclear factor kappa B (NF-κB) also plays a key role in the process of releasing inflammatory mediators in the activated M1-type microglia, which is thought to be the main regulator of the M1 phenotype (17-20). From this perspective, to regulate the function of microglial cells by targeting Nrf2 and/or NF-κB with active compounds may help to prevent inflammation-mediated neurotoxicity.
Achyranthes bidentata Bl. (A. bidentata) is a traditional herbal medicine, which has been used in China for thousands of years, mainly for strengthening muscles and bones. A. bidentata polypeptide (ABPP) is one of the active ingredients extracted from A. bidentata, our previous studies demonstrated that ABPP could promote nerve regeneration and protect ischemic brain injury (21-23). Achyranthes bidentata polypeptide k (ABPPk) was the excellent neuroprotective component isolated from ABPP by high performance liquid chromatography (HPLC), and it was demonstrated that ABPPk could be beneficial to ischemic stroke and Parkinson’s disease of rats (24-26).
Previously, we have reported that ABPPk could reduce NO production, inhibit NF-κB activation, and suppress the infiltration of polymorphonuclear neutrophils after ischemic stroke in rats, implying that ABPPk could potentially prevent the neuroinflammation after ischemia (25). However, whether the signaling pathways involved in the neuroinflammation can be interfered by ABPPk is still unknown. Therefore, in this study, we investigate the effect of ABPPk in LPS-induced BV2 microglia inflammatory response, and further explore whether Nrf2 plays a key role in the anti-inflammatory effect of ABPPk. The study will provide clear evidence for anti-neuroinflammation of ABPPk in the use of neuroprotection.
Methods
Materials
LPS (Escherichia coli O111:B4) was purchased from Sigma (St. Louis, MO, USA). Cell counting kit-8 was purchased from Dojindo (Kumamoto, Japan). Enzyme-linked immunosorbent assay (ELISA) kits for TNF-α, IL-6 and prostaglandin E2 (PGE2) were purchased from Novus biological (Littleton, CO, USA), Bosterbio (Pleasanton, CA, USA) and R&D Systems (Minneapolis, MN, USA), respectively. Griess reagent and glutathione (GSH)-Glo™ Glutathione assay kit were obtained from Promega (Madison, WI, USA). Specific primary antibodies for inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), p-NF-κB (p65), HO-1, Nrf2, β-actin and Lamin B were all purchased from Abcam (San Francisco, CA, USA). Protein extraction kit, nuclear extraction kit, bicinchoninic acid assay (BCA) protein assay kit and enhanced chemiluminescence (ECL) Western Blotting Substrate were all obtained from Thermo Fisher Scientific (Waltham, MA, USA). BV2 microglia were purchased from the Institute of Basic Medical Sciences of the China Science Academy. Nrf2 siRNA, control siRNA, siRNA transfection reagent and siRNA transfection medium were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ABPPk was isolated and purified as described previously (24,27).
BV2 microglia culture
BV2 microglia were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA), 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco, Carlsbad, CA, USA) in a 5% CO2 incubator at 37 °C. Cells were cultured in 75 cm2 filter-capped flasks and passaged every two days. After confluence, cells were digested with 0.25% trypsin-EDTA and then planted in different culture plates or dishes according to the required density.
Cell viability assay
BV2 microglia were planted at the density of 2×105 cells/mL in 96-well culture plates. Twenty-four hours later, cells were pretreated with different concentrations of ABPPk (0.008, 0.04, 0.2, 1 and 5 µg/mL) for 30 min, followed by LPS stimulation (1 µg/mL) for 24 h. Then 10 µL CCK-8 was added to each well and incubated at 37 °C water bath in dark for 2 h. Absorbance was determined at the wavelength of 450 nm by using enzyme-linked immunodetector, which indirectly reflected the cell viability.
Nitrite measurement
BV2 microglia were planted in a 6-well plate at a density of 2×105 cells/mL. Twenty-four hours later, cells were pretreated with ABPPk for 30 min and followed by LPS stimulation for 24 h. The supernatants of culture were collected and centrifuged for measuring NO production by Griess reagent assay kit according to manufacturer’s instructions.
Measurement of TNF-α, IL-6 and PGE2 production
BV2 microglia were planted in a 6-well plate at a density of 2×105 cells/mL. Twenty-four hours later, cells were treated with ABPPk for 30 min prior to stimulation with LPS. After 24 h, the supernatants of culture were collected and centrifuged. Concentrations of TNF-α, IL-6 and PGE2 were measured with their respective ELISA kits according to the manufacturer’s instructions.
GSH-Glo™ glutathione assay
BV2 microglia were planted in a 96-well plate at a density of 2×105 cells/mL. After 24 h, cells were treated with ABPPk and incubate for the following 24 h. At the end of experiment, levels of GSH for each group were measured by using GSH-Glo™ Glutathione assay kit according to the manufacturer’s instructions. Luminescence was then read with luminometer.
Immunofluorescence
Following treatments, BV2 microglia on coverslips were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature and later washed three times for 5 min with phosphate buffered saline (PBS). After permeabilization and blocking with PBS containing 5% bovine serum albumin (BSA) and 0.1% Triton X-100, coverslips were incubated overnight with the primary p-NF-κB (p65) antibody (2.5 µg/mL) and Nrf2 antibody (5 µg/mL) at 4 °C, then with the secondary antibody for 2 h at room temperature after washing with PBS. Then, coverslips were incubated for 5 min with 50 nM DAPI (Invitrogen, Carlsbad, CA, USA) for nuclei staining, and mounted on microscope slides with glycergel mounting medium (Dako, Bucks, UK) for photographing under fluorescent microscope. Representative fluorescence images were obtained using Axio Imager M2 fluorescence microscope (Zeiss, German).
Western blot
Cell lysates and nuclear lysates of BV2 microglia were extracted using extraction kits according to the manufacturer’s instructions, respectively. Protein concentrations were determined by Pierce™ BCA protein assay kit. Proteins were electrophoresed on 10% SDS-polyacrylamide gel and transferred onto a PVDF membrane (Millipore, Bedford, MA, USA). The membranes were blocked in 5% non-fat milk for 1 h at room temperature and incubated with the specific primary antibodies (iNOS, 1:1,000; COX-2, 1:1,000; p-NF-κB (p65), 1:1,000; HO-1, 1:1,000; Nrf2, 1:1,000; β-actin, 1:3,000; Lamin B, 1:1,000) overnight at 4 °C. The membranes were incubated with HRP-conjugated secondary antibody for 1 h at room temperature. The proteins were then detected with ECL Western Blotting Substrate. Quantitative analysis of Western blot was performed by ImageJ software (NIH Image, Washington, DC, USA).
Nrf2 siRNA transfection
BV2 microglia were planted in 6-well culture plates at a certain density and cultured in a 5% CO2 incubator at 37 °C. After the cells were confluent to over 80%, they were transfected with Nrf2 siRNA or control siRNA using siRNA transfection reagent according to the instructions with some modification. In brief, for each well, the cells were washed twice with anti-biotic and serum free siRNA transfection medium, and then the culture medium was replaced with 0.8 mL siRNA transfection medium before transfection. Four µL of siRNA duplex and 4 µL of siRNA transfection reagent were diluted with 96 µL of siRNA transfection medium in two separate Eppendorf tubes, respectively. Then mix the two dilute solutions very gently and incubate the mixture at room temperature for 30 min. Then the mixture was added into cell culture medium. Cells were incubated for 6 h at 37 °C in a 5% CO2 incubator. After that, culture medium was changed to complete medium and incubated for another 24 h. Transfection efficiency was determined by western blot for expression level of Nrf2 protein.
Statistical analysis
Data in this study were represented as mean ± standard deviation (SD) of three independent experiments and compared using one-way analysis of variance (ANOVA) followed by a post hoc Student Newman-Keuls test (multiple comparisons). The GraphPad Prism 6 software package (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. P<0.05 was considered as significant difference.
Results
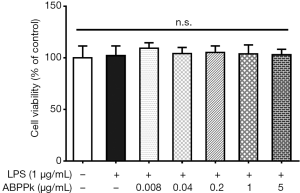
ABPPk does not affect the viability of BV2 microglia
Cell counting kit-8 was used to investigate the effect of ABPPk on BV2 cell viability. As shown in Figure 1, ABPPk had no effect on cell viability in the range from 0.008 to 5 µg/mL. Therefore, 0.04, 0.2, 1 and 5 µg/mL of ABPPk were used in the following experiments.
ABPPk reduces NO and PGE2 production in LPS-activated BV2 microglia
LPS stimulation resulted in a significant increase in NO production in BV2 microglia compared with untreated control cells. Pretreatment with ABPPk (0.04–5 µg/mL) for 30 min reduced NO production significantly in a concentration-dependent manner (P<0.01) (Figure 2A). Western blot analysis showed that pretreatment with 0.2–5 µg/mL of ABPPk reduced expression level of iNOS protein after LPS stimulation significantly (P<0.05) (Figure 2B), suggesting that ABPPk could reduce iNOS-mediated NO production in LPS-activated microglia.
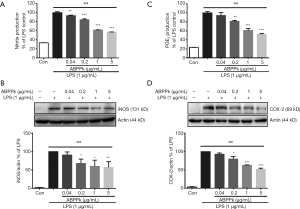
LPS stimulation also led to a significant increase of PGE2 production in BV2 microglia compared to untreated control cells (Figure 2C). However, pretreated with 0.2–5 µg/mL ABPPk suppressed the production of PGE2 significantly (P<0.01). Based on this, we further detected the expression level of COX-2 protein in LPS stimulated BV2 microglia with or without ABPPk pretreatment. As shown in Figure 2D, LPS stimulation resulted in a remarkable increase in COX-2 protein expression. However, pretreatment with 0.2 to 5 µg/mL of ABPPk produced an obvious inhibitory effect on LPS-induced COX-2 protein expression (P<0.05). These results suggested that ABPPk could inhibit the increase of COX-2-mediated PGE2 in LPS-activated microglia.
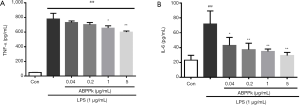
ABPPk reduces TNF-α and IL-6 production in LPS-activated BV2 microglia
After LPS stimulation, the concentration of TNF-α secreted into the supernatant of culture medium increased significantly (Figure 3A). However, pretreatment with 1 and 5 µg/mL of ABPPk led to a significant reduction of TNF-α concentration in the LPS-stimulated cells (P<0.05). We also examined the effect of ABPPk on IL-6 levels in LPS-activated microglia. The results showed that LPS stimulation caused the increase of IL-6 concentration produced by activated microglia, while ABPPk pretreatment significantly reduced the increase of IL-6 concentration (Figure 3B).
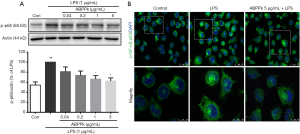
ABPPk inhibits activation of NF-κB in LPS-activated BV2 microglia
As an important transcription factor, the activation of the NF-κB initiates and regulates the expression of several inflammatory processes during inflammation which are attributed to the pathology of neurodegenerative diseases (28). Our previous study showed that ABPPk could suppress the activation of NF-κB in the ischemic brain of rats (25). Here we investigated whether ABPPk could inhibit the activation of NF-κB in LPS-activated microglial. As shown in Figure 4A, LPS stimulation significantly increased the activation of NF-κB compared with untreated control cells. However, preconditioning with ABPPk reduced the activation of NF-κB induced by LPS, and the effect was concentration-dependent. Immunofluorescence results also showed that ABPPk reduces the translocation to nucleus of NF-κB (Figure 4B).
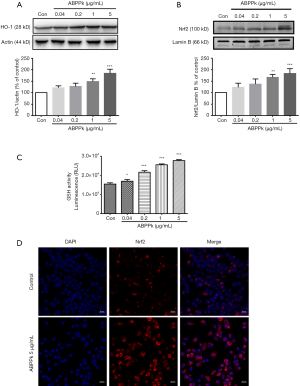
ABPPk up-regulates HO-1 and Nrf2 protein expression in BV2 microglia
Some studies have shown that the Nrf2/HO-1 signaling pathway is involved in NF-κB activation and production of inflammatory mediators (29,30). In this study, we also explored whether Nrf2/HO-1 antioxidant pathway was involved in the anti-inflammatory effect of ABPPk. Western blot results showed that treatment with 0.04 and 0.2 µg/mL ABPPk had no significant effects on HO-1 and Nrf2 expression levels in BV2 microglia. However, on increasing the concentration of ABPPk to 1 µg/mL, there were significant and concentration dependent up-regulations in HO-1 and Nrf2 protein levels (Figure 5A,B). The concentration-dependent increase of GSH levels in BV2 microglia after ABPPk treatment was also confirmed by GSH-Glo™ Glutathione Assay (Figure 5C). Based on the above results, we further used immunofluorescent staining to detect whether ABPPk could promote Nrf2 translocation to the nucleus. Staining results showed that Nrf2 expression was significantly increased in ABPPk treated microglia (Figure 5D). The result was consistent with the results of western blot.
ABPPk exerts anti-inflammatory effect through activating Nrf2
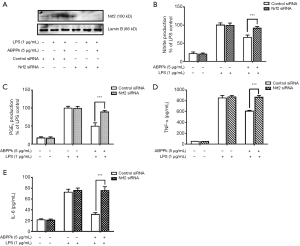
In order to determine whether Nrf2 is involved in the anti-inflammatory mechanism of ABPPk, Nrf2 was knockdown in BV2 microglia by siRNA. As shown in Figure 6A, Western blot result showed that the Nrf2 siRNA successfully interfered with the expression of Nrf2 in BV2 microglia. Furthermore, we stimulated the Nrf2-knockdown BV2 microglia by LPS, and measured the levels of NO, PGE2, TNF-α and IL-6 produced by the cells with or without ABPPk pretreatment. The results showed that in Nrf2-knockdown BV2 microglia, 5 µg/mL of ABPPk pretreatment did not reduce the production of NO, PGE2, TNF-α and IL-6, in other words, the inhibitory effect of ABPPk on NO, PGE2, TNFα, and IL-6 production seemed to be reversed by Nrf2 knockdown (Figure 6B,C,D,E).
Discussion
This present study demonstrated that ABPPk can exert anti-inflammatory effect by inhibiting NF-κB and activating Nrf2 signaling pathways of BV2 microglia. In LPS-induced neuroinflammatory response, ABPPk can reduce the production of inflammatory mediators through Nrf2 dependent mechanism.
Activation of microglia plays crucial roles in neuroinflammation in the central nervous system (2). LPS is a component of the outer wall of Gram-negative bacteria cell wall, which is composed of lipids and polysaccharides (glycolipids). As an endotoxin, LPS can activate the toll-like receptor 4 (TLR4) on the membrane of microglia (31,32). TLRs are important in transducing extracellular signals into intracellular cascade molecular and triggering the inflammatory responses (33,34). In neuroinflammation-related injuries, TRL4/NF-κB signaling pathway can accelerate the progress of inflammation (35,36). Besides NF-κB, other signaling pathways such as downstream IL-1 receptor kinase (IRAK) (37), tumor necrosis factor receptor-related factor 6 (TRAF-6) (38,39) and MAPK are all involved in promoting the secretion of various pro-inflammatory mediators (40-44).
Previously we have shown that ABPPk can inhibit the activation of NF-κB in the brain after ischemia (25). The activated p-NF-κB (p65) subunit translocated from cytoplasm to nucleus and triggered the expression of downstream proteins, including iNOS, COX-2, and IL-6, etc. (45,46). In this study, ABPPk reduced the production of NO and PGE2 in LPS-activated BV2 microglia (see Figures 2,3). Further experiments showed that ABPPk could reduce the increased iNOS and COX-2 expression levels induced by LPS stimulation, indicating that the inhibitory effect of ABPPk on NO and PGE2 production may probably be related to its capability of inhibiting iNOS and COX-2 expression. ABPPk also reduced TNF-α and IL-6 production in LPS-activated BV2 microglia (Figure 3). The activated p-NF-κB (p65) was highly expressed in the nucleus of BV2 microglia after LPS stimulation, whereas ABPPk inhibited the phosphorylation and translocation of p65 (see Figure 4). These results suggest that ABPPk reduces pro-inflammatory mediators’ production by inhibiting the phosphorylation and translocation to the nuclear of p-NF-κB (p65) subunit.
The regulation of microglial polarization from pro-inflammatory M1-type to anti-inflammatory M2-type appears as an outstanding therapeutic approach for neurodegenerative diseases (7). There are plenty of studies show that some compounds that inhibit the activation of NF-κB can also activate Nrf2 (47,48). The antioxidant transcription factor Nrf2 is a key regulator in resistant to peroxidation damage and inflammation (11,49). Nrf2 can activate its downstream antioxidant protein HO-1, thus inhibiting NADPH oxidase, reducing the release of ROS in microglial cells, thereby attenuating oxidative stress and its mediated neuronal loss (50). Because of the advantages of multi-target and low toxicity, natural products as the modulators of microglial phenotypes have attracted considerable concerns in the therapy of neurodegenerative diseases (51). Many natural compounds have been shown to have anti-inflammatory effects through activating Nrf2 and/or inhibiting NF-κB signaling pathways. For example, Sinomenine can inhibit inflammatory response by acting on Nrf2/HO-1 and NF-κB signaling pathways (52). Hesperetin can attenuate LPS-induced neuroinflammation by regulating TRL4/NF-κB signaling pathway (48). Icariin can inhibit microglia-mediated neuroinflammation by targeting Nrf2 signaling (13). Our previous studies have confirmed that ABPPk has a potent neuroprotective effect and shows some anti-inflammatory effects. We are interested to see if ABPPk can also exert its anti-inflammatory effects by regulating NF-κB and/or Nrf2. Based on the inhibition of ABPPk on the production of anti-inflammatory factors in LPS-activated BV2 microglia, we examined the effect of ABPPk on Nrf2/HO-1 expression level in BV2 microglia (see Figure 5A,B). The results implied that ABPPk increases the expression levels of Nrf2/HO-1 in BV2 microglia. Not only that, measurement of endogenous levels of GSH also showed that ABPPk increases the level of GSH in BV2 microglia as shown in Figure 5C. Once activation, Nrf2 translocates to the nucleus and bind to the antioxidant response elements to induce the expression of anti-oxidant related genes (53,54). Further immunofluorescent staining showed that ABPPk enhanced the nuclear translocation of Nrf2 as shown in Figure 5D. The above results show that ABPPk can enhance the expression and transcriptional activity of Nrf2, suggesting that ABPPk may also play an anti-inflammatory role through Nrf2.
In order to elucidate the direct relationship between anti-inflammatory effect of ABPPk and activation of Nrf2, we transfected BV2 cells with mouse Nrf2 siRNA to investigate whether ABPPk still had anti-inflammatory effect on BV2 cells with Nrf2 gene knocked down. Western blot confirmed the success of Nrf2 knockdown. No matter adding LPS stimulation or ABPPk treatment, the interfered BV2 microglia had almost no Nrf2 protein expression (see Figure 6A). Then we used ABPPk to treat the interfered BV2 microglia, and LPS stimulation was applied to both the interfered and non-interfered cells, and the supernatant was collected separately to detect the content of pro-inflammatory factors. The results showed that the content of pro-inflammatory factors produced by the Nrf2 knocked down BV2 microglia was almost the same as that of the LPS stimulation group. It seemed that Nrf2 knockout eliminated the inhibitory effects of ABPPk on the production of pro-inflammatory mediators in LPS-activated microglia as shown in Figure 6B,C,D,E. This result confirms our hypothesis that Nrf2 is indeed a key signaling molecule for ABPPk to play an anti-inflammatory role. In future studies, we may conduct more studies on the neuroprotective mechanisms of ABPPk and Nrf2.
Conclusions
This study demonstrated the anti-inflammatory effect of ABPPk on LPS-stimulated microglia inflammation, which may contribute to ABPPk’s neuroprotective effect. Further evidence suggests that the anti-inflammatory effect of ABPPk is mediated by the activation of Nrf2, providing a new target for the development of ABPPk as a neuroprotective agent with clear mechanisms.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China, No.81871554, 81501058; the Natural Science Foundation of Jiangsu Province, Grant No. BK20161285; and a grant from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Kabba JA, Xu Y, Christian H, et al. Microglia: housekeeper of the central nervous system. Cell Mol Neurobiol 2018;38:53-71. [Crossref] [PubMed]
- Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 2017;35:441-68. [Crossref] [PubMed]
- Ransohoff RM, Schafer D, Vincent A, et al. Neuroinflammation: ways in which the immune system affects the brain. Neurotherapeutics 2015;12:896-909. [Crossref] [PubMed]
- Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 2016;53:1181-94. [Crossref] [PubMed]
- Song GJ, Suk K. Pharmacological modulation of functional phenotypes of microglia in neurodegenerative diseases. Front Aging Neurosci 2017;9:139. [Crossref] [PubMed]
- Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 2016;142:23-44. [Crossref] [PubMed]
- Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci 2018;12:306. [Crossref] [PubMed]
- Ma L, Niu W, Lv J, et al. PGC-1α-mediated mitochondrial biogenesis is involved in cannabinoid receptor 2 agonist AM1241-induced microglial phenotype amelioration. Cell Mol Neurobiol 2018;38:1529-37. [Crossref] [PubMed]
- Shu ZM, Shu XD, Li HQ, et al. Ginkgolide B protects against ischemic stroke via modulating microglia polarization in mice. CNS Neurosci Ther 2016;22:729-39. [Crossref] [PubMed]
- Wang Y, Huang Y, Xu Y, et al. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal 2018;28:141-63. [Crossref] [PubMed]
- Okorji UP, Velagapudi R, El-Bakoush A, et al. Antimalarial drug artemether inhibits neuroinflammation in BV2 microglia through Nrf2-dependent mechanisms. Mol Neurobiol 2016;53:6426-43. [Crossref] [PubMed]
- Shah SA, Khan M, Jo MH, et al. Melatonin stimulates the SIRT1/Nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-induced oxidative stress to rescue postnatal rat brain. CNS Neurosci Ther 2017;23:33-44. [Crossref] [PubMed]
- Zheng Y, Zhu G, He J, et al. Icariin targets Nrf2 signaling to inhibit microglia-mediated neuroinflammation. Int Immunopharmacol 2019;73:304-11. [Crossref] [PubMed]
- Liu S, Li G, Tang H, et al. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci Lett 2019;709:134386. [Crossref] [PubMed]
- Bahar E, Kim JY, Yoon H. Quercetin attenuates manganese-induced neuroinflammation by alleviating oxidative stress through regulation of apoptosis, iNOS/NF-κB and HO-1/Nrf2 pathways. Int J Mol Sci 2017. [Crossref]
- Wang Y, Zhao CS. Sigma-1 receptor activation ameliorates LPS-induced NO production and ROS formation through the Nrf2/HO-1 signaling pathway in cultured astrocytes. Neurosci Lett 2019;711:134387. [Crossref] [PubMed]
- Taetzsch T, Levesque S, McGraw C, et al. Redox regulation of NF-κB p50 and M1 polarization in microglia. Glia 2015;63:423-40. [Crossref] [PubMed]
- Shi X, Cai X, Di W, et al. MFG-E8 selectively inhibited Aβ-induced microglial M1 polarization via NF-κB and PI3K-Akt pathways. Mol Neurobiol 2017;54:7777-88. [Crossref] [PubMed]
- Liu Q, Zhang Y, Liu S, et al. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J Neuroinflammation 2019;16:10. [Crossref] [PubMed]
- Xiang B, Xiao C, Shen T, et al. Anti-inflammatory effects of anisalcohol on lipopolysaccharide-stimulated BV2 microglia via selective modulation of microglia polarization and down-regulation of NF-κB p65 and JNK activation. Mol Immunol 2018;95:39-46. [Crossref] [PubMed]
- Yuan Y, Shen H, Yao J, et al. The protective effects of Achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Res Bull 2010;81:25-32. [Crossref] [PubMed]
- Shen H, Yuan Y, Ding F, et al. Achyranthes bidentata polypeptides confer neuroprotection through inhibition of reactive oxygen species production, Bax expression, and mitochondrial dysfunction induced by overstimulation of N-methyl-D-aspartate receptors. J Neurosci Res 2010;88:669-76. [PubMed]
- Cheng Q, Yuan Y, Sun C, et al. Neurotrophic and neuroprotective actions of Achyranthes bidentata polypeptides on cultured dorsal root ganglia of rats and on crushed common peroneal nerve of rabbits. Neurosci Lett 2014;562:7-12. [Crossref] [PubMed]
- Yu S, Wang C, Cheng Q, et al. An active component of Achyranthes bidentata polypeptides provides neuroprotection through inhibition of mitochondrial-dependent apoptotic pathway in cultured neurons and in animal models of cerebral ischemia. PLoS One 2014;9:e109923. [Crossref] [PubMed]
- Cheng Q, Tong F, Shen Y, et al. Achyranthes bidentata polypeptide k improves long-term neurological outcomes through reducing downstream microvascular thrombosis in experimental ischemic stroke. Brain Res 2019;1706:166-76. [Crossref] [PubMed]
- Peng S, Wang C, Ma J, et al. Achyranthes bidentata polypeptide protects dopaminergic neurons from apoptosis in Parkinson's disease models both in vitro and in vivo. Br J Pharmacol 2018;175:631-43. [Crossref] [PubMed]
- Cheng Q, Jiang C, Wang C, et al. The Achyranthes bidentata polypeptide k fraction enhances neuronal growth in vitro and promotes peripheral nerve regeneration after crush injury in vivo. Neural Regen Res 2014;9:2142-50. [Crossref] [PubMed]
- Shabab T, Khanabdali R, Moghadamtousi SZ, et al. Neuroinflammation pathways: a general review. Int J Neurosci 2017;127:624-33. [Crossref] [PubMed]
- Wu PS, Ding HY, Yen JH, et al. Anti-inflammatory activity of 8-hydroxydaidzein in LPS-stimulated BV2 microglial cells via activation of Nrf2-antioxidant and attenuation of Akt/NF-κB-inflammatory signaling pathways, as well as inhibition of COX-2 activity. J Agric Food Chem 2018;66:5790-801. [Crossref] [PubMed]
- Dwivedi S, Rajasekar N, Hanif K, et al. Sulforaphane ameliorates okadaic acid-induced memory impairment in rats by activating the Nrf2/HO-1 antioxidant pathway. Mol Neurobiol 2016;53:5310-23. [Crossref] [PubMed]
- Jiao FZ, Wang Y, Zhang HY, et al. Histone deacetylase 2 inhibitor CAY10683 alleviates lipopolysaccharide induced neuroinflammation through attenuating TLR4/NF-κB signaling pathway. Neurochem Res 2018;43:1161-70. [Crossref] [PubMed]
- Shi H, Wang XL, Quan HF, et al. Effects of betaine on LPS-stimulated activation of microglial M1/M2 phenotypes by suppressing TLR4/NF-κB pathways in N9 cells. Molecules 2019. [Crossref]
- Azam S, Jakaria M, Kim IS, et al. Regulation of toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol 2019;10:1000. [Crossref] [PubMed]
- Rahimifard M, Maqbool F, Moeini-Nodeh S, et al. Targeting the TLR4 signaling pathway by polyphenols: a novel therapeutic strategy for neuroinflammation. Ageing Res Rev 2017;36:11-9. [Crossref] [PubMed]
- Liu J, Chen Q, Jian Z, et al. Daphnetin protects against cerebral ischemia/reperfusion injury in mice via inhibition of TLR4/NF-κB signaling pathway. Biomed Res Int 2016;2016:2816056. [PubMed]
- Michel HE, Menze ET. Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-κB and activating Nrf2 and PPAR-γ signaling pathways. Eur J Pharmacol 2019;857:172422. [Crossref] [PubMed]
- Aslanidis A, Karlstetter M, Scholz R, et al. Activated microglia/macrophage whey acidic protein (AMWAP) inhibits NFκB signaling and induces a neuroprotective phenotype in microglia. J Neuroinflammation 2015;12:77. [Crossref] [PubMed]
- Cui Y, Wang Y, Zhao D, et al. Loganin prevents BV-2 microglia cells from Aβ1-42 -induced inflammation via regulating TLR4/TRAF6/NF-κB axis. Cell Biol Int 2018;42:1632-42. [Crossref] [PubMed]
- Li M, Zhang D, Ge X, et al. TRAF6-p38/JNK-ATF2 axis promotes microglial inflammatory activation. Exp Cell Res 2019;376:133-48. [Crossref] [PubMed]
- Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 2016;173:649-65. [Crossref] [PubMed]
- Hoogland ICM, Westhoff D, Engelen-Lee JY, et al. Microglial activation after systemic stimulation with lipopolysaccharide and Escherichia coli. Front Cell Neurosci 2018;12:110. [Crossref] [PubMed]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia 2013;61:71-90. [Crossref] [PubMed]
- Chen Z, Jalabi W, Shpargel KB, et al. Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci 2012;32:11706-15. [Crossref] [PubMed]
- Jo SH, Kim ME, Cho JH, et al. Hesperetin inhibits neuroinflammation on microglia by suppressing inflammatory cytokines and MAPK pathways. Arch Pharm Res 2019;42:695-703. [Crossref] [PubMed]
- Shi S, Liang D, Chen Y, et al. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer's disease. Eur J Immunol 2016;46:665-76. [Crossref] [PubMed]
- Rim HK, Cho W, Sung SH, et al. Nodakenin suppresses lipopolysaccharide-induced inflammatory responses in macrophage cells by inhibiting tumor necrosis factor receptor-associated factor 6 and nuclear factor-κB pathways and protects mice from lethal endotoxin shock. J Pharmacol Exp Ther 2012;342:654-64. [Crossref] [PubMed]
- Chen J, Yin W, Tu Y, et al. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: involvement of NF-κB inhibition and Nrf2 pathway activation. Eur J Pharmacol 2017;806:1-9. [Crossref] [PubMed]
- Muhammad T, Ikram M, Ullah R, et al. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 2019. [Crossref]
- Velagapudi R, El-Bakoush A, Olajide OA. Activation of Nrf2 pathway contributes to neuroprotection by the dietary flavonoid tiliroside. Mol Neurobiol 2018;55:8103-23. [Crossref] [PubMed]
- Xiong W, MacColl Garfinkel AE, Li Y, et al. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest 2015;125:1433-45. [Crossref] [PubMed]
- Jin X, Liu MY, Zhang DF, et al. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol Res 2019;145:104253. [Crossref] [PubMed]
- Wu Y, Lin Z, Yan Z, et al. Sinomenine contributes to the inhibition of the inflammatory response and the improvement of osteoarthritis in mouse-cartilage cells by acting on the Nrf2/HO-1 and NF-κB signaling pathways. Int Immunopharmacol 2019;75:105715. [Crossref] [PubMed]
- Onasanwo SA, Velagapudi R, El-Bakoush A, et al. Inhibition of neuroinflammation in BV2 microglia by the biflavonoid kolaviron is dependent on the Nrf2/ARE antioxidant protective mechanism. Mol Cell Biochem 2016;414:23-36. [Crossref] [PubMed]
- Park SY, Kim YH, Park G. Cucurbitacins attenuate microglial activation and protect from neuroinflammatory injury through Nrf2/ARE activation and STAT/NF-κB inhibition. Neurosci Lett 2015;609:129-36. [Crossref] [PubMed]


