
Role of immunotherapy in metastatic renal cell cancer: past, present and future
Introduction
It was only in the late 90s when the link between the immunology and oncology was known to scientists. Tumor shrinkage of sarcoma following injection of killed bacteria noted by Coley was one of the earlier observations which led to considering immunotherapy in cancer treatment (1). Although the initial studies on interferon gamma-1b failed to improve outcomes in metastatic renal cell carcinoma (mRCC) (2), cases of spontaneous regression of metastatic lung lesions perpetuated investigations to study the potential role of immunotherapy in mRCC (3).
RCC accounts for 2–3% of all malignancies, and it constitute >80% of primary renal neoplasm. Clear cell type is the commonest subtype of RCC (85%). Approximately, 1/3rd of cases diagnosed with RCC have metastasis at presentation, and another 1/3rd usually develop metastases at follow up (4). When localized, surgical excision can be curative. Unfortunately, prognosis and outcome of mRCC remains dismal.
Staging and risk assessment
Like in most of the solid tumors, TNM staging system is used in RCC to define the anatomic extent of cancer spread and to stratify to stage groups. The variable clinical course of patients in the same stage, however, forced researchers to think beyond the TNM staging to prognosticate outcomes. Subsequently, various parameters such as disease extent, tumor size, tumor necrosis, nuclear grade histology, performance status, and presence of paraneoplastic syndromes (e.g., anemia, hypercalcemia, thrombocytosis, fever, weight loss) have been used to formulate an improved prognostic model (5-7). Most recent and frequently used models are the stage, size, grade and necrosis (SSIGN) score and the University of California Los Angeles Integrated Staging System (UISS) (8,9).
Predicting risk in advanced disease
In 1999, Motzer et al. published a multivariate analysis study on mRCC cases. They formulated a risk assessment scale of mRCC patients during pre-targeted therapy era (10). Parameters used in this model were low Karnofsky performance status, high serum lactate dehydrogenase, low hemoglobin, high ‘‘corrected’’ serum calcium, and absence of prior nephrectomy. Later in 2005, Mekhail et al. validated Motzer’s model, and identified two additional independent prognostic factors: prior radiotherapy and sites of metastasis (11). While both models were helpful in prognosticating patients with mRCC, in the current era of targeted therapy, they are not frequently used.
Currently, most clinicians use the model proposed by the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model for prognostication of survival in patients with mRCC (12). The six parameters used in “IMDC score” are anemia, thrombocytosis, neutrophilia, hypercalcemia, Karnofsky performance status, and <1 year from diagnosis to first-line targeted therapy. IMDC model has since undergone external validation by several groups (12-14).
Cytokines in advanced RCC
Interleukins (IL) and interferons
IL-2 is a cytokine that primarily acts directly on T cells and plays a role in adequate functioning of the immune system. Similarly, interferon-alpha stimulates T cells to attack cancer cells. Both these agents were studied for their efficacy in several randomized controlled trials (15,16). However, both agents individually were found to be effective only in selected patients. More importantly, drug toxicity limited its use.
Many trials were embarked to explore the utility of adjunctive agents such as NG-monomethyl-L-arginine, superoxide dismutase, and others in reducing the toxicity of these cytokines without compromising the efficacy (17,18). However, with the shift to immunotherapeutic and targeted therapy, their role in the setting of more effective and better tolerated checkpoint inhibitors and anti-angiogenic agents is undefined but it still could be an option in selected patients.
Basics immune pathways
Programmed death-1 (PD-1)/programmed death ligand-1 (PD-L1) pathway
This pathway contains two proteins called PD-1, and PD-L1. There is a differential expression of these proteins on different cells. Cancer cells usually express PD-L1 and immune cells express PD-1. Interaction between PD-1 and PD-L1 forms an immune evading “shield” which protects tumor cells from being attacked by the immune system.
Cytotoxic T-lymphocyte associated protein 4 (CTLA-4) Pathway
Similarly, in CTLA-4 pathway, CTLA-4 and cluster of differentiation 28 (CD28; a cell membrane protein), which are both expressed on T cells, compete with each other to bind to B7, a membrane protein present on activated antigen presenting cell (APC). The amount of CTLA-4:B7 binding compared to CD28:B7 binding determines if the T cells will be activated.
New ray of hope: combination therapies
Recently many studies have been conducted to study the efficacy of combination therapies. Various targets like PD-1, PD L-1, CTLA-4, VEGF, and mTOR have been studied and we will be discussing various trials and their outcome in following sections.
Nivolumab plus ipilimumab vs. sunitinib
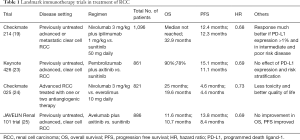
Amongst the initial landmark trials on immunotherapy for mRCC, the Bristol-Myers Squibb sponsored trial. Motzer et al. published the results of the phase 3 trial (Check Mate 214 trial, ClinicalTrials.gov number, NCT02231749). In this study, one arm received nivolumab plus ipilimumab (550 cases untreated mRCC) whereas other arm was given sunitinib (546 cases). They found that combination arm had significant overall survival (OS) and objective response rate (ORR) benefit compared to sunitinib alone. However, progression free survival (PFS) was similar in both the arms. Interestingly, within nivolumab plus ipilimumab arm, a greater response was noted in PD-L1 positive tumors when compared to PD-L1 negative ones (19,20). Cella et al. studied the same cohort and reported fewer adversities and better health-related quality of life (HRQoL) with the combination therapy (21).
Pembrolizumab plus axitinib vs. sunitinib
While Bristol-Myers Squibb was working on nivolumab plus ipilimumab combination regimen, Merck Sharp & Dohme Corp. initiated phase 1b trial (ClinicalTrials.gov, number NCT02133742) to assess the safety of treatment combination of pembrolizumab plus axitinib (22).
With the success of phase 1b, combination regimen entered phase 3 trial (KEYNOTE-426, ClinicalTrials.gov Identifier: NCT0285333). This phase 3 open-label study randomized 861 participants to either pembrolizumab plus axitinib (432 patients) or sunitinib alone (429 patients). At follow up, combination arm was superior to sunitinib alone with regards to OS [90% vs. 78% at 12 months, hazard Ratio (HR) 0.53, 95% CI, 0.38–0.74] and PFS (median 15.1 vs. 11.1 months, HR 0.69, 95% CI, 0.57–0.84), and ORR (59% vs. 36%, P<0.001) (23).
Two differences to note between Check Mate 214 trial and KEYNOTE-426 trial are the treatment responses based on risk stratification (IMDC) and PD-L1 expression. While Check Mate 214 trial reported better outcome to combination regimen with intermediate- or poor-risk disease or with PD-L1-expressing tumors, the KEYNOTE-426 trial reported benefit irrespective of PD-L1 expression and risk classification. However, the response duration and off-treatment survival for the pembrolizumab plus axitinib therapy are still being tabulated, and the study is expected to be completed by January 27, 2020.
Based on results of the above two landmark trials, FDA has approved nivolumab plus ipilimumab combination and pembrolizumab plus axitinib on April 16, 2018 and on April 19, 2019, respectively.
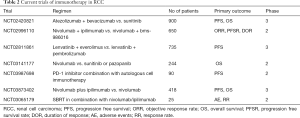
Other major landmark trials for use of immunotherapy in RCC are highlighted in Table 1. Current ongoing clinical trials studying the use of immunotherapy in RCC are highlighted in Table 2.
Full table
Full table
Avelumab plus axitinib vs. sunitinib
After the successful outcome of KEYNOTE-426 and Check Mate 214 trials, many other pharmaceutical companies have started studying other PD1/PD-L1 inhibitors. A phase 3, multinational, randomized study compared avelumab (a PD-L1 inhibitor) plus axitinib with sunitinib alone in advanced RCC (JAVELIN Renal 101 trial, ClinicalTrials.gov number, NCT02684006). They found significant longer median PFS in general (13.8 vs. 8.4 months, HR 0.69) and among those with PD-L1 positive expressing tumors (13.8 vs. 7.2 months, HR 0.61) (25). However, there was no benefit in OS among the two groups. Also, both groups had similar grade ≥3 toxicities.
Based on this study, the FDA, on May 14, 2019, approved avelumab plus axitinib as first-line treatment of mRCC.
Atezolizumab plus bevacizumab vs. sunitinib
Results of phase 2 study (funded by F Hoffmann-La Roche Ltd. and Genentech Inc.) which confirmed efficacy and safety of combination regimen of atezolizumab plus bevacizumab (IMmotion150 trial, ClinicalTrials.gov number, NCT01984242) was shared at the ASCO meeting in 2017.
Following this, a multicenter, open-labelled, phase 3 trial was conducted involving 915 patients with previously untreated mRCC (IMmotion151 trial, ClinicalTrials.gov, number NCT02420821). One arm received atezolizumab plus bevacizumab (454 patients) and other arm received sunitinib alone (461 patients) (26).
The combination arm had improved PFS as compared to sunitinib alone arm in the PD-L1 positive population (HR 0.74; 95% CI, 0.57–0.96; P=0.0217). Based on the available data on interim analysis, no significant difference in the median OS in the intent-to-treat population was noted amongst the two groups (26).
Sunitinib or pazopanib plus nivolumab
CheckMate 016 trial (Clinicaltrials.gov identifier, NCT01472081) was a phase 1 study which involved studying combination of sunitinib with nivolumab or Pazopanib with nivolumab. Unfortunately, both these combinations resulted in high-grade toxicities leading to premature closure of study. All patients in both the arms (100%) suffered treatment-related adverse events (AEs). 82% in sunitinib plus nivolumab arm and 70% in Pazopanib plus nivolumab arm had grade 3/4 treatment-related AEs (27).
Pazopanib and pembrolizumab
Similar to CheckMate 016 trial, another phase I/II study (Novartis sponsored) was discontinued prematurely due to liver toxicity (Pazopanib and pembrolizumab) (28).
Immune-check inhibitor as a monotherapy
As mentioned above, most of the trials initially focused on PD-1/PD L-1 inhibitor–based combination therapy. With encouraging results of combination therapy, PD-1 inhibitor monotherapy as a first line agent was studied in KEYNOTE-427 trial.
This trial studied pembrolizumab monotherapy as first-line therapy in aRCC (ClinicalTrials.gov Identifier: NCT02853344) and found promising efficacy and acceptable tolerability (27). Best response was noted amongst patient with intermediate-risk/poor-risk disease and with expression of PD-L1 of ≥1 percent (29).
Other ongoing trials
There is an ongoing phase III trial on combination therapy including lenvatinib comparing with sunitinib alone (NCT02811861). Another study is comparing nivolumab plus cabozantinib vs. sunitinib (NCT03141177). Results of these trials might add more immunotherapeutic options in the treatment of RCC (Table 2).
Current data of immunotherapy as second line agent (study on nivolumab vs. everolimus)
It is clear that immunotherapeutic agents targeting PD1/PD-L1 and CTLA 4 have proven their benefit as a first-line agent in mRCC.
Subsequently, trials studied their role as a second-line agent in previously treated aRCC (24,30). One of them was Bristol-Myers Squibb sponsored Check Mate 025 trial which included 803 patients who underwent randomization to nivolumab (406 patients) or everolimus (397 patients). There was a median OS benefit of 5.4 months with nivolumab as compared to everolimus (25.0 vs. 19.6 months). Similarly, nivolumab arm had a better ORR and PFS as compared to everolimus. Importantly, nivolumab arm had better hazard ratio for death (from any cause) as compared to everolimus (HR 0.73; 98.5% CI, 0.57–0.93; P=0.002). When compared for grade 3 or more AEs, nivolumab was found to be better than everolimus (19% vs. 37%) (24).
Escudier et al. also studied the same cohort of patients for any difference in OS and ORR with nivolumab compared with everolimus based on multiple prognostic factors. They found nivolumab to be superior to everolimus across all the risk groups, age groups, and consistently better irrespective to the number and sites of metastases, and type and duration of prior therapy. Also, both PD L1 positive and negative population had similar response to nivolumab (30).
Cella et al. studied the HRQoL differences in patients receiving nivolumab vs. everolimus (31). HRQoL was assessed based on Functional Assessment of Cancer Therapy-Kidney Symptom Index-Disease Related Symptoms (FKSI-DRS) and European Quality of Life (EuroQol)-5 Dimensions (EQ-5D) questionnaires. HRQoL improvement was noted to be significantly higher with nivolumab compared to everolimus (95% CI, 1.4–1.9; P<0.0001).
Role of immunotherapy as adjuvant therapy
Currently, there is no benefit of using targeted therapy or immunotherapy as adjuvant therapy after definitive surgical resection, as these approaches have shown no OS benefit and, instead, have led to increased toxicity.
Personalized, patient-specific immunotherapeutic approach
Autologous dendritic cell (DC)-based immunotherapy (AGS-003 product)
A single-arm, open-label phase 2 trial (sponsored by Argos Therapeutics, ClinicalTrials.gov Identifier, NCT00678119), studied the effectiveness of autologous DC therapy vs. sunitinib in aRCC patients with unfavorable risk factors (32). The concept behind this study was to use genetic engineering technique and follow an autologous immunotherapy approach. They generated AGS-003 product which was an ex vivo prepared concentrate of DCs co-electroporated with the patient’s amplified tumor RNA and synthetic CD40L RNA. Subsequently, this patient-tailored, RNA-loaded mature DCs concentrate is injected intradermal in to an axillary lymph node. These DCs were designed to efficiently present tumor antigens to both CD4+ and CD8+ T cells and thereby heightening the anti-tumor response of the patient’s immune system. Study population involved 21 intermediate and poor-risk mRCC patients who received serial intradermal injections of AGS-003 following debulking nephrectomy and sunitinib until disease progression. This combination therapy showed median PFS of 11.2 months (95% CI, 6.0–19.4 months) and a median OS of 30.2 months (95% CI, 9.4–57.1 months) (32).
Unfortunately, a phase III trial that compared the combination therapy to standard therapy failed to show any benefit, which led to premature termination of the study (ADAPT trial, NCT01582672) (33).
Cancer vaccine IMA901
Another phase 3 trial sponsored by Immatics Biotechnologies GmbH (IMPRINT trial, ClinicalTrials.gov Identifier: NCT01265901), investigated the clinical effect of adding IMA901, a multi-peptide cancer vaccine (TUMAP), to sunitinib (33). 339 HLA-A*02-positive, newly diagnosed and untreated aRCC patients were randomly assigned to either sunitinib plus serial intradermal vaccinations of IMA901 (204 patients) or sunitinib alone (135 patients). Unfortunately, median OS did not differ significantly between the groups (HR 1.34; 95% CI, 0.96–1.86; P=0.087) (34).
Conclusions
Immune-checkpoint inhibitors (ICI) have revolutionized the treatment of many cancer types, including RCC. Specific checkpoint inhibition has already become a primary treatment modality in the management of advanced disease. There are several ongoing trials to better elucidate the ideal, personalized strategy, and it is expected that treatment of RCC would witness the addition of newer immunotherapeutic drugs. As usage of these newer molecules are increasing, reports of the AEs are also on rising trend (35,36). The challenge is to develop therapies which would be more impactful than the existing ones with minimal AEs.
Acknowledgments
The authors thank to Dr. John G Park, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN, USA (Park.John@mayo.edu) for his help to critically review the manuscript for accuracy.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 1991.3-11. [PubMed]
- Negrier S, Escudier B, Lasset C, et al. Recombinant Human Interleukin-2, Recombinant Human Interferon Alfa-2a, or Both in Metastatic Renal-Cell Carcinoma. N Engl J Med 1998;338:1272-8. [Crossref] [PubMed]
- Vogelzang NJ, Priest ER, Borden L. Spontaneous regression of histologically proved pulmonary metastases from renal cell carcinoma: a case with 5-year followup. J Urol 1992;148:1247-8. [Crossref] [PubMed]
- Schmidinger M, Hejna M, Zielinski CC. Aldesleukin in advanced renal cell carcinoma. Expert Rev Anticancer Ther 2004;4:957-80. [Crossref] [PubMed]
- Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol 2002;20:4559-66. [Crossref] [PubMed]
- Yaycioglu O, Roberts WW, Chan T, et al. Prognostic assessment of nonmetastatic renal cell carcinoma: a clinically based model. Urology 2001;58:141-5. [Crossref] [PubMed]
- Sekulic M, Martin S, Lal A, et al. Intravascular Large B-Cell Lymphoma of the Kidney. Kidney Int Rep 2018;3:1501-5. [Crossref] [PubMed]
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 2003;97:1663-71. [Crossref] [PubMed]
- Patard JJ, Kim HL, Lam JS, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol 2004;22:3316-22. [Crossref] [PubMed]
- Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530-40. [Crossref] [PubMed]
- Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005;23:832-41. [Crossref] [PubMed]
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794-9. [Crossref] [PubMed]
- Ko JJ, Xie W, Kroeger N, et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015;16:293-300. [Crossref] [PubMed]
- Wells JC, Stukalin I, Norton C, et al. Third-line Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2017;71:204-9. [Crossref] [PubMed]
- Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 1995;13:688-96. [Crossref] [PubMed]
- Motzer RJ, Bacik J, Murphy BA, et al. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002;20:289-96. [Crossref] [PubMed]
- Kilbourn RG, Fonseca GA, Trissel LA, et al. Strategies to reduce side effects of interleukin-2: evaluation of the antihypotensive agent NG-monomethyl-L-arginine. Cancer J Sci Am 2000;6 Suppl 1:S21-30. [PubMed]
- Samlowski WE, Petersen R, Cuzzocrea S, et al. A nonpeptidyl mimic of superoxide dismutase, M40403, inhibits dose-limiting hypotension associated with interleukin-2 and increases its antitumor effects. Nat Med 2003;9:750-5. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Akella P, Loganathan S, Jindal V, et al. Anti PD-1 immunotherapy related interstitial lung disease presenting as respiratory failure - A review with case series. Respir Med Case Rep 2018;26:17-22. [Crossref] [PubMed]
- Cella D, Grunwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019;20:297-310. [Crossref] [PubMed]
- Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol 2018;19:405-15. [Crossref] [PubMed]
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1116-27. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380:1103-15. [Crossref] [PubMed]
- Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019;393:2404-15. [Crossref] [PubMed]
- Amin A, Plimack ER, Ernstoff MS, et al. Safety and efficacy of nivolumab in combination with sunitinib or pazopanib in advanced or metastatic renal cell carcinoma: the CheckMate 016 study. J Immunother Cancer 2018;6:109. [Crossref] [PubMed]
- Chowdhury S, McDermott DF, Voss MH, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 2017;35:4506. [Crossref]
- McDermott DF, Lee J-L, Szczylik C, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): Results from cohort A of KEYNOTE-427. J Clin Oncol 2018;36:4500. [Crossref]
- Escudier B, Sharma P, McDermott DF, et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur Urol 2017;72:962-71. [Crossref] [PubMed]
- Cella D, Grunwald V, Nathan P, et al. Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:994-1003. [Crossref] [PubMed]
- Amin A, Dudek AZ, Logan TF, et al. Survival with AGS-003, an autologous dendritic cell-based immunotherapy, in combination with sunitinib in unfavorable risk patients with advanced renal cell carcinoma (RCC): Phase 2 study results. J Immunother Cancer 2015;3:14. [Crossref] [PubMed]
- Figlin RA, Wood CG. ADAPT Study Group. ADAPT: An ongoing international phase III randomized trial of autologous dendritic cell immunotherapy (AGS-003) plus standard treatment in advanced renal cell carcinoma (RCC). J Clin Oncol 2014;32:449. [Crossref]
- Rini BI, Stenzl A, Zdrojowy R, et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2016;17:1599-611. [Crossref] [PubMed]
- Mishra AK, Sahu KK, James A. Disseminated herpes zoster following treatment with benralizumab. Clin Respir J 2019;13:189-91. [Crossref] [PubMed]
- Sahu KK, Mishra A, Chastain I. Novel anticancers and dermatological adversities: old rivals but new challenges. BMJ Case Rep 2018. [Crossref] [PubMed]


