
Prognostic effect of allogeneic hematopoietic stem cell transplantation on first and non-first complete remission in acute myeloid leukemia
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered the cure for intermediate or adverse-risk acute myeloid leukemia (AML) (1). It offers patients longer overall survival (OS) (2-4). Unfortunately, human leukocyte antigen (HLA) matched related donor is unavailable in more than 70% patients. Some of these patients, may experience relapse and subsequently achieve CR (non-CR1) with treatment. Some non-CR1 patients eventually receive allo-HSCT. It is unclear whether allo-HSCT will improve the survival of these patients.
We collected 53 AML patients from The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/) to determine whether prognosis was different between CR1 and non-CR1 AML patients following allo-HSCT (5). All patients were between ages 22 and 69, registered between November 2001 and March 2010. Non-CR1 was defined as complete remission after two or more relapse treatments. These data were used to assess prognostic criteria according to the recent European Leukemia Net (ELN) classification (6). OS was the study endpoint and was defined as the time from diagnosis to death or censoring at the last follow-up.
Clinical characteristics of the CR1 group (n=32) and the non-CR1 group (n=21) were summarized in Table 1. The median age of the CR1 group was significantly older than non-CR1 group (57.5 vs. 48, P=0.031); the CR1 group also had more patients older than 60 years (23 vs. 3, P=0.014).There were more favorable-risk patients in the non-CR1 group (P<0.001), No significant differences was found in the type of donor between CR1 and non-CR1groups.
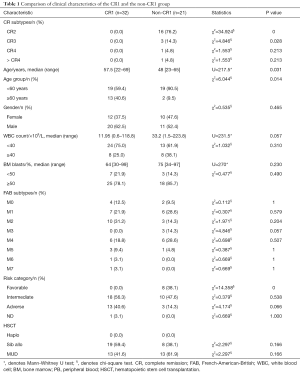
Full table
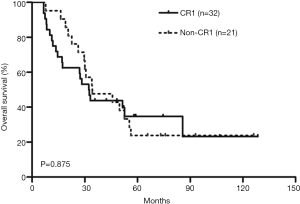
Kaplan-Meier survival curves showed that there was no significant difference in OS between the CR1 and non-CR1 groups (Figure 1). We also assessed the prognostic significance of clinical and molecular characteristic, including remission status, risk category, age, peripheral WBC count, FLT3-ITD, NPM1and DNMT3A with multivariate analysis (Table 2). The results indicated that none of these factors had any impact on OS.
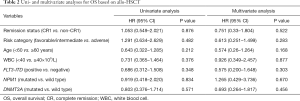
Full table
We included the clinical features with significant differences between the two groups in Table 1 and typical prognostic factors into a multivariate analysis, and found no significant difference in OS between the CR1 group and the non-CR1 group (Table 2).
In this study, we found that there was no prognostic difference between CR1 and non-CR1 patients following allo-HSCT. Allo-HSCT might weaken the adverse effect of relapse in non-CR1 patients and should still be offered once consequential remission was achieved.
The optimum timing for allo-HSCT in AML patients remains controversial. A previous study found that AML patients who did not receive a transplant in CR1, but underwent allo-HSCT in CR2 had superior survival compared with the patients who did not underwent allo-HSCT, particularly in the intermediate-risk patients (7). Compared with this study, our research found no difference in adult AML patients who underwent allo-HSCT in CR1 or subsequent CR, providing some insight into the timing to perform transplantation.
Our study was limited by its small sample size. The favorable risk patients are exclusively included in non CR1 series because were considered for allogeneic HSCT only in case of AML relapse. Furthermore, the baseline characteristics of the CR1 group and the non-CR1 group were not balanced, with older patients in the CR1 group, and more good-risk patients in the non-CR1 group, which could have affected the results. Nevertheless, our study showed same prognosis in CR1 and non-CR1 patients following allo-HSCT, and supported that allo-HSCT was a reasonable post-remission therapy in adult AML patients who had reached another CR after relapse from CR1.Further studies with larger cohorts are warranted to validate our findings.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81500118, 61501519).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell 2014;14:275-91. [Crossref] [PubMed]
- Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009;301:2349-61. [Crossref] [PubMed]
- Cornelissen JJ, van Putten WL, Verdonck LF, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood 2007;109:3658-66. [Crossref] [PubMed]
- Kurosawa S, Yamaguchi T, Miyawaki S, et al. A Markov decision analysis of allogeneic hematopoietic cell transplantation versus chemotherapy in patients with acute myeloid leukemia in first remission. Blood 2011;117:2113-20. [Crossref] [PubMed]
- Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059-74. [Crossref] [PubMed]
- Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424-47. [Crossref] [PubMed]
- Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 2013;31:1293-301. [Crossref] [PubMed]


