
The proportion of CD19+CD24hiCD27+ regulatory B cells predicts the occurrence of acute allograft rejection in liver transplantation
Introduction
Acute allograft rejection (AR) still remains a major cause of early allograft loss and an obstacle for long-term allograft survival after liver transplantation, despite the usage of modern immunosuppressive agents. Thus, the early diagnosis and treatment of AR are very important for patients undergoing transplantation. Although the percutaneous liver biopsy is currently preferred as the gold standard diagnosis for AR, still it is an invasive tool with a risk of related complications post biopsy. Despite the increasing interest in the study of potential biomarkers and risk factors for AR, few of these factors are used routinely in clinical practice (1-3). Currently, the immunological monitoring after liver transplantation relies completely on clinical evaluation and the measurement of serum immunosuppressant levels. Pro-inflammatory and immunoregulatory cytokines are the most studied biomarkers of AR (4). However, many of these cytokines are unable to differentiate between AR and infections.
While B cells play an important role in the pro-inflammatory immune responses, the immunoregulatory roles of B cells have also been reported recently (5). A subset of IL-10-secreting B cells with regulatory functions, the so-called regulatory B cells (Bregs), have been identified in recent years. Emerging evidence has shown the critical role of Bregs in regulating various diseases including autoimmune diseases, inflammation, chronic infections, cancer and graft rejection (6-9).
In humans, CD19+CD24hiCD38hi transitional Bregs (tBregs) and CD19+CD24hiCD27+ memory Bregs (mBregs) are two subsets of Bregs enriched for IL-10 expression. In patients with systemic lupus erythematosus, tBregs were defective in IL-10 expression and unable to suppress helper T cell 1 (Th1) responses in vitro. By contrast, another study showed increased IL-10 expression in human mBregs from patients with autoimmune disorders. Several studies have reported a decreased number of Bregs in renal transplant patients with graft rejection (10,11). However, little is known about the relationship between the number of Bregs and AR in patients post liver transplantation.
In the present study, the perioperative number and proportion of Bregs were tracked in patients undergoing liver transplantation with or without AR.
Methods
Patients
Nineteen patients received liver allografts from cardiac death (DCD) donors, who were included in this retrospective study. A biopsy of allograft tissues with Banff score ≥5 was used to further confirm the diagnosis of AR in patients with postoperative elevated transaminases and suspected AR. The kinetic changes of the perioperative proportion of Bregs were retrospectively analyzed in patients diagnosed with AR (AR group, n=5) and other patients with stable allograft liver function (SF group, n=10) from Jan. 2015 to Dec. 2016 in our single center. Additionally, proportions of Breg in four AR recipients were suspected to be present but eventually diagnosed with hemolytic or obstructive jaundice were properly analyzed. This study was performed in accordance with the recommendations of the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University with written informed consent from all subjects. All subjects submitted written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University (approval number 2015-SRFA-095).
All patients received conventional immunosuppressive agents after liver transplantation, including tacrolimus, cyclosporin A, mycophenolate mofetil (MMF) and steroids (prednisone). The dose of tacrolimus was adjusted to maintain the serum concentration at 5–10 ng/mL. Patients with HBV infection received nucleoside analogs plus hepatitis B immunoglobulin (HBIG) as prophylaxis. The clinical data and proportion of Bregs at different time points starting from 0 (pre-transplantation), 1, 2, 4, 6 and 8 weeks post-transplantation were collected and evaluated for analysis.
Flow cytometric analysis
Fresh peripheral blood after lysis of red blood cells was surface stained with monoclonal antibodies purchased from Biolegend for flow cytometer assay (tBregs: CD19-APC-Cy7, CD24-Percp-Cy5.5 & CD38-FITC; mBregs: CD19-APC-Cy7, CD24-Percp-Cy5.5 & CD27-FITC). After cells were incubated with cell surface antibodies for 30 minutes at 4 °C in the dark, they were washed with PBS and then analyzed by flow cytometer. Sample acquisition was performed by a CATON II (BD Bioscience) and data were analyzed with FlowJo software (TreeStar).
Statistical analysis
The results are shown as the mean ± SD. Two-way analysis of variance was used to compare the mean proportions of mBregs or tBregs across different time points and different groups. A two-sample t-test was used to compare the mean proportions of mBregs or tBregs in the different groups at each time point. All analyses were performed using Stata software (version 11.0, StataCorp LLC, College Station, Texas, USA). P values less than 0.05 (two-tailed) was considered statistically significant.
Results
Kinetic changes in the CD19+CD24hiCD27+ Bregs of liver transplant recipients with stable allograft liver function and AR
Initially, we analyzed the proportion of Bregs in patients with stable allograft liver function after liver transplantation. Figure 1 show a representative plot demonstrating tBregs as a CD19+CD24hiCD38hi population and mBregs as a CD19+CD24hiCD27+ population. We investigated both the tBregs and mBregs at different time points including pre- and post-liver transplantation. No significant changes were found regarding both the tBreg and mBreg proportions across all time points in ten patients with stable allograft function (Figure 2A,B, SF group).
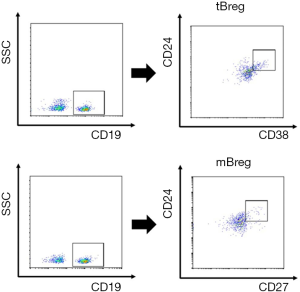
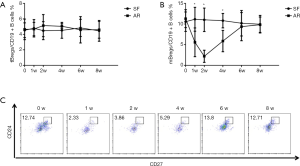
The clinical data and proportions of Bregs were analyzed in five patients developed AR as indicated by persistently elevated serum levels of bilirubin, glutamic pyruvic transaminase (ALT) and glutamic oxalacetic transaminase (AST). A biopsy of allograft tissues with Banff score ≥5 was used to further confirm the diagnosis of AR. The clinical characteristics of the five patients are listed in Table 1. As shown in Figure 2A, the frequencies of tBregs did not significantly change post transplantation. In contrast, the frequencies of mBregs decreased significantly in all five patients diagnosed with AR (Figure 2B,C).

Full table
All five patients received anti-rejection therapies immediately post the diagnosis of AR, such as glucocorticoid pulse therapy and immunosuppressant modulation as shown in Table 1. Interestingly, all the AR patients responded well to the treatments. Of note, the proportions of mBregs increased significantly after treatment with the anti-rejection therapies. Normal levels of mBregs were detected at 8 weeks post-transplantation in all these patients.
The mBreg cell frequencies in the differential diagnosis of jaundice post transplantation
We also questioned whether a postoperative decrease in the proportion of mBregs could help to specifically predict the occurrence of AR. Four recipients experienced abnormal postoperative jaundice in our study, two of whom were diagnosed with hemolytic jaundice, and the other two were diagnosed with obstructive jaundice due to anastomotic stenosis. As shown in Figure 3A,B, unlike the results in the AR patients, the proportion of mBregs did not decrease in any of the four patients.

Discussion
AR is an important problem in post-liver transplantation, which may significantly impair liver function and long-term allograft survival. However, the risk factors of AR remain largely unknown, and early diagnosis is difficult. Our present study shows that decreased frequencies of mBregs are associated with an increased incidence of AR occurrence. The mBregs may also function as a key mediator of anti-rejection therapies. To the best of our knowledge, this is the first study to indicate the predictive role of mBregs in acute liver rejection.
B cells have important functions in mediating the rejection of transplanted organs by producing donor-specific antibodies, secreting cytokines, and presenting antigens to T cells. However, B cells with regulatory properties, which are termed Bregs, have attracted increasing interest in various diseases including transplantation. With the regulatory functions, Bregs are involved in repressing inflammation (12,13), inhibiting autoimmune disorders (14,15), and inducing transplant tolerance (16,17). B cell depletion by rituximab promoted acute graft rejection in patients undergoing renal transplantation, which was potentially due to the loss of Bregs (18). In contrast, the adoptive transfer of Bregs resulted in an expansion of regulatory T cells and alleviated symptoms of experimental autoimmune encephalomyelitis (19).
While the effector T or memory B cells during the early post transplantation period were unaffected by immunosuppressive, the Treg cells were significantly suppressed (20). Several studies have shown the effects of immunosuppressants on Bregs. Adoptive transfer of IL-10+ regulatory B cells decreases myeloid-derived macrophages in the central nervous system in a transgenic amyotrophic lateral sclerosis model (21). Tebbe et al. found that calcineurin inhibitors reduced the number of Bregs and the IL-10 production of Bregs in both renal transplant recipients and non-transplanted healthy subjects (22). In contrast, rapamycin decreased the development of bronchiolitis obliterans by inhibiting pro-inflammatory cytokines and increasing the infiltration of Bregs (23). However, no remarkable changes in Breg subsets were found in patients post liver transplantation in our study.
Although larger numbers of studies have shown the critical roles of Bregs in a wide variety of inflammatory conditions, the phenotype of Bregs has yet to be elucidated. Besides IL-10, many other surface markers have been used for identifying Bregs. Thus, different subsets of Bregs have been reported among different experimental settings both in mice and humans. In addition, whether and how subsets of Bregs are developmentally linked still remain unknown. In the present study, we mainly focused on the CD19+CD24hiCD38hi transitional B cells and CD19+CD24hiCD27+ memory B cells. However, the changes and functions of other subsets of Bregs such as CD19+CD25hiCD71hi Br1 cells were not analyzed and may need to be further studied.
Multiple Bregs cell subsets with many similar phenotypes and effector regulatory functions have been described (24,25). In humans, both CD19+CD24hiCD38hi Bregs (26) and CD19+CD24hiCD27+ Bregs (27) have been identified. Both CD19+CD24hiCD38hi transitional B cells and CD19+CD24hiCD27+ memory B cells are enriched for IL-10 expression. Cherukuri A’s group found that in patients with graft rejection, CD24hiCD38hi transitional B cells had increased TNF-α expression (1). Patients with drug-free long-term kidney graft function display increased numbers of peripheral B cells, particularly CD19+IgD-CD38+CD27+ memory Bregs (28). An association between decreased Breg frequencies and cGVHD severity was reported. Patients with chronic graft-versus-host disease had fewer CD24hiCD27+ B cells and IL-10-producing CD24hiCD27+ B cells (29). Another study demonstrated that granzyme B-producing Bregs were significantly decreased in rheumatoid arthritis patients, suggesting that the impairment of this Breg subset was correlated with the pathogenesis of rheumatoid arthritis (30). A recent prospective study found that tBregs are associated with protection from kidney AR, indicating that transitional B cells may be a biomarker and therapeutic intervention in renal transplantation (2). However, in the present study, we found that CD19+CD24hiCD27+ mBregs, but not CD19+CD24hiCD38hi tBregs, were significantly decreased in all five patients diagnosed with AR. Interestingly, the proportion of mBregs increased markedly during the recovery period when anti-rejection therapy was initiated, indicating that the mBregs may function as a key mediator of anti-rejection therapies. Inconsistent with our findings, the proportion of CD19+CD24hiCD27+ B cells was significantly lower in patients with Graves’ disease than in healthy individuals. These Breg proportions were restored to normal levels in recovered patients (31).
The early diagnosis of AR is critical for treatment and patient outcomes. Continued jaundice post transplantation is an important sign for AR post liver transplantation. We found that a decrease in the proportion of mBregs was not detected in the four patients with jaundice that was not caused by AR.
However, due to the low incidence of acute AR post liver transplantation, the number of patients with AR presented in this study is relatively low. This study was limited by a small sample size, which impaired the ability to draw a definitive conclusion about the role of mBregs in predicting the acute liver rejection. Studies with a larger sample size should be conducted to further confirm these preliminary findings.
In conclusion, our study suggested for the first time that a decrease in CD19+CD24hiCD27+ mBregs may be correlated with an increased AR incidence among patients post liver transplantation. However, the sensitivity and specificity of mBregs in predicting acute liver rejection need to be further studied.
Acknowledgments
We thank Prof. Liwei Lu (The University of Hong Kong) for helpful advice.
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81771716, 81571557, 8197061257, 81600450), Jiangsu Province “333” Project (BRA2016515), the Key Talent’s Subsidy Project in “Science and Education Strong Health Project of Jiangsu Province” and 863 Young Scientists Special Fund grant SS2015AA0209322.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was carried out in accordance with the recommendations of the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the First Affiliated Hospital of Nanjing Medical University (approval number 2015-SRFA-095).
References
- Sood S, Haifer C, Yu L, et al. A novel immune function biomarker identifies patients at risk of clinical events early following liver transplantation. Liver Transpl 2017;23:487-97. [Crossref] [PubMed]
- Dollinger MM, Plevris JN, Bouchier IA, et al. Peripheral eosinophil count both before and after liver transplantation predicts acute cellular rejection. Liver Transpl Surg 1997;3:112-7. [Crossref] [PubMed]
- Hickman PE, Potter JM, Pesce AJ. Clinical chemistry and post-liver-transplant monitoring. Clin Chem 1997;43:1546-54. [PubMed]
- Germani G, Rodriguez-Castro K, Russo FP, et al. Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol 2015;21:1061-8. [Crossref] [PubMed]
- Firl DJ, Benichou G, Kim JI, et al. A Paradigm Shift on the Question of B Cells in Transplantation? Recent Insights on Regulating the Alloresponse. Front Immunol 2017;8:80. [Crossref] [PubMed]
- Yang M, Rui K, Wang S, et al. Regulatory B cells in autoimmune diseases. Cell Mol Immunol 2013;10:122-32. [Crossref] [PubMed]
- Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 2017;14:662-74. [Crossref] [PubMed]
- Chong AS, Sciammas R. Matchmaking the B-cell signature of tolerance to regulatory B cells. Am J Transplant 2011;11:2555-60. [Crossref] [PubMed]
- Nouël A, Simon Q, Jamin C, et al. Regulatory B cells: an exciting target for future therapeutics in transplantation. Front Immunol 2014;5:11. [Crossref] [PubMed]
- Cherukuri A, Rothstein DM, Clark B, et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-α expression ratio in regulatory B cells. J Am Soc Nephrol 2014;25:1575-85. [Crossref] [PubMed]
- Shabir S, Girdlestone J, Briggs D, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant 2015;15:1384-91. [Crossref] [PubMed]
- Horikawa M, Weimer ET, DiLillo DJ, et al. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol 2013;190:1158-68. [Crossref] [PubMed]
- Ray A, Dittel BN. Mechanisms of Regulatory B cell Function in Autoimmune and Inflammatory Diseases beyond IL-10. J Clin Med 2017. [Crossref] [PubMed]
- Matsushita T, Horikawa M, Iwata Y, et al. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol 2010;185:2240-52. [Crossref] [PubMed]
- Ellis JS, Braley-Mullen H. Mechanisms by Which B Cells and Regulatory T Cells Influence Development of Murine Organ-Specific Autoimmune Diseases. J Clin Med 2017. [Crossref] [PubMed]
- Lal G, Nakayama Y, Sethi A, et al. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation 2015;99:1817-28. [Crossref] [PubMed]
- Ding Q, Yeung M, Camirand G, et al. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest 2011;121:3645-56. [Crossref] [PubMed]
- Clatworthy MR, Watson CJ, Plotnek G, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med 2009;360:2683-5. [Crossref] [PubMed]
- Pennati A, Ng S, Wu Y, et al. Regulatory B Cells Induce Formation of IL-10-Expressing T Cells in Mice with Autoimmune Neuroinflammation. J Neurosci 2016;36:12598-610. [Crossref] [PubMed]
- Kim HY, Cho ML, Jhun JY, et al. The imbalance of T helper 17/regulatory T cells and memory B cells during the early post-transplantation period in peripheral blood of living donor liver transplantation recipients under calcineurin inhibitor-based immunosuppression. Immunology 2013;138:124-33. [Crossref] [PubMed]
- Pennati A, Asress S, Glass JD, et al. Adoptive transfer of IL-10+ regulatory B cells decreases myeloid-derived macrophages in the central nervous system in a transgenic amyotrophic lateral sclerosis model. Cell Mol Immunol 2018;15:727-30. [Crossref] [PubMed]
- Tebbe B, Wilde B, Ye Z, et al. Renal Transplant Recipients Treated with Calcineurin-Inhibitors Lack Circulating Immature Transitional CD19+CD24hiCD38hi Regulatory B-Lymphocytes. PLoS One 2016;11:e0153170. [Crossref] [PubMed]
- Zhao Y, Gillen JR, Meher AK, et al. Rapamycin prevents bronchiolitis obliterans through increasing infiltration of regulatory B cells in a murine tracheal transplantation model. J Thorac Cardiovasc Surg 2016;151:487-96.e3. [Crossref] [PubMed]
- Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015;42:607-12. [Crossref] [PubMed]
- Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012;30:221-41. [Crossref] [PubMed]
- Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010;32:129-40. [Crossref] [PubMed]
- Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117:530-41. [Crossref] [PubMed]
- Pallier A, Hillion S, Danger R, et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 2010;78:503-13. [Crossref] [PubMed]
- de Masson A, Bouaziz JD, Le BH, et al. CD24(hi)CD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood 2015;125:1830-9. [Crossref] [PubMed]
- Xu L, Liu X, Liu H, et al. Impairment of Granzyme B-Producing Regulatory B Cells Correlates with Exacerbated Rheumatoid Arthritis. Front Immunol 2017;8:768. [Crossref] [PubMed]
- Zha B, Wang L, Liu X, et al. Decrease in proportion of CD19+ CD24(hi) CD27+ B cells and impairment of their suppressive function in Graves' disease. PLoS One 2012;7:e49835. [Crossref] [PubMed]


