Efficacy and safety of mecapegfilgrastim for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer: a randomized, multicenter, active-controlled phase III trial
Introduction
Although the advances in targeted therapy and immune therapy, chemotherapy still plays a critical role in the cancer treatment strategy. Meanwhile, chemotherapy induced toxicities can adversely affect the patients’ tolerance to chemotherapy and limit the effectiveness of chemotherapy. Neutropenia is a common complication, study shows that 65.5% of patients experienced a proven hematological toxicity with grade 3–4 neutropenia when treated with docetaxel, doxorubicin and cyclophosphamide (TAC) treatment regimen (1). Moreover, febrile neutropenia (FN) can be life-threatening, which associated with high risk of mortality.
The recombinant human granulocyte colony-stimulating factor (rhG-CSF), filgrastim and pegfilgrastim have been widely used for the prevention of chemotherapy-induced neutropenia (2-5). Filgrastim possesses rapid renal clearance and requires daily administration during chemotherapy. Pegfilgrastim, as the long-acting rhG-CSF, has comparable efficacy and safety profile to filgrastim, but its longer half-life allows once-per-cycle administration during chemotherapy (6,7). Therefore, pegfilgrastim could offer great convenience, which could enable better patient compliance and improved clinical outcomes (8,9). But in China, the US- and EU-approved pegfilgrastim (Neulasta) is not available, leaving the short-acting G-CSF as the major treatment option.
Mecapegfilgramtim (code name HHPG-19K), a long-acting rhG-CSF, has been developed by covalently bonding a 19-kDa polyethylene glycol (PEG) to the N terminus of filgrastim. The previous phase II study has shown that mecapegfilgrastim was well tolerated in non-small cell lung carcinoma (NSCLC) patients receiving platinum-based chemotherapy, the dose of 100 µg/kg was recommended for efficacy evaluating, and the mean plasma half-life value was 55.99 hours (10). The following phase III study showed that once-per-cycle injection of mecapegfilgrastim was as effective and safe as daily filgrastim for prophylaxis of chemotherapy-induced neutropenia in NSCLC patients (11).
For clinically evaluating all G-CSF medicines, patients with (neo)adjuvant breast cancer represent a sensitive population (12). It provides a homogenous patient population which means that they exhibit less inter-patient variation in terms of potential for treatment related toxicity and other confounding factors. Multiple randomized clinical studies have been conducted to demonstrate equivalence between biosimilar and reference G-CSF in breast cancer. In a phase II trial, mecapegfilgrastim preliminarily presented better clinical efficacy as the secondary prophylactic therapy for neutropenia and equal tolerance compared with G-CSF in one cycle treatment in breast cancer patients, and a dose of 100 µg/kg was recommended for further study (13).
In this study, we performed a randomized phase III study in patients with breast cancer. The primary objective was to further evaluate the non-inferiority of mecapegfilgrastim compared with filgrastim as the primary prophylactic therapy during the first cycle of chemotherapy with respect to duration of severe neutropenia (DSN), and also to demonstrate whether the fixed 6 mg dose showed a similar safety and efficacy to the weight-based dose of 100 µg/kg. This trial was approved by the National Medical Products Administration of China (registration number: 2010L00501) and registered on ClinicalTrials.gov (NCT01611051).
Methods
Patients
The patients who met the following criteria were enrolled: pathologically confirmed and previously untreated breast cancer who were eligible to receive neoadjuvant or adjuvant chemotherapy defined by the study protocol; age ranging from 18 to 70 years old; body weight ≥45 kg; Eastern Cooperative Oncology Group (ECOG) performance status 0–1; expected tolerance of chemotherapy ≥4 cycles; adequate organ function: (I) normal bone marrow hematopoietic function without bleeding tendency [international normalized ratio (INR) <1.5]; (II) adequate hematologic function: hemoglobin ≥90 g/L, white blood cell (WBC) ≥4.0×109/L, absolute neutrophil count (ANC) ≥2.0×109/L, platelet count (PLT) ≥100×109/L; (III) adequate renal and hepatic function; (IV) no cardiopulmonary dysfunction; negative pregnancy test (blood sample or urine sample) within 7 days prior to enrollment for child bearing age females who are willing to use reliable contraception methods during the study.
The exclusion criteria include: had a history of bone marrow or stem-cell transplantation; had acute or active infection and received systemic antibiotics within 72 hours before chemotherapy; had hematologic disease could affect bone marrow function; underwent pregnancy or breast feeding; had been enrolled into other clinical trials within 4 weeks before randomization into this study; had previously received pegfilgrastim treatment; hypersensitive to PEG-rhG-CSF or rhG-CSF or other biological agents; had previously received systemic chemotherapy, definitive radiotherapy, palliative radiotherapy within 4 weeks; some special cases that the researchers determined not eligible for the study.
Study design
This was a randomized, open-label, active-control, multicenter study. The eligible patients received either anthracyclines-taxane (AT) chemotherapy (epirubicin 75 mg/m2 combined with docetaxel 75 mg/m2) or adriamycin and cyclophosphamide (AC) chemotherapy (epirubicin 100 mg/m2 combined with cyclophosphamide 600 mg/m2) on day 1 of each cycle and every 3 weeks for up to 4 cycles, except for disease progression or unacceptable toxicity.
For cycle 1, the patients were randomized at a ratio of 1:1:1 to receive a single dose of mecapegfilgrastim 100 µg/kg or a 6 mg fixed dose on day 3 (≥48 hours after chemotherapy), or filgrastim 5 µg/kg/day since day 3 (≥48 hours after chemotherapy), continuing until a documented ANC ≥5.0×109/L twice or ANC ≥15×109/L once after the expected nadir, or for up to 14 days, whichever occurred first.
For cycles 2–4, the patients in mecapegfilgrastim groups continued to receive mecapegfilgrastim 100 µg/kg or a 6 mg fixed dose on day 3 in each cycle. Patients in the control group only received filgrastim treatment in cycle 1.
Mecapegfilgrastim was provided by Hengrui Medicine Co., Ltd. (Lianyungang, China) and short-acting filgrastim was provided by Kyowa Hakko Kirin China Pharmaceutical Co., Ltd. (Shanghai, China).
Blood monitoring
In cycle 1, blood samples were collected within 24 h of the initiation of chemotherapy and on day 1, 3, 5, 7, 8, 9, 10, 11, 13, 15, 17 and 21 during cycle 1, or until an ANC ≥2.0×109/L was reached.
In cycles 2 to 4, blood samples were taken on day 5, 7, 9, 11, 13 and 21 of each cycle. ANC assessments during cycles 2 to 4 were performed within 24 h of chemotherapy, on day 5, 7, 9, 11, 13 and 21 of each cycle, until an ANC ≥2.0×109/L was achieved.
Endpoints
The primary endpoint was the mean duration of grade ≥3 neutropenia (defined as ANC <1.0×109/L) during cycle 1 of chemotherapy. The secondary endpoints included the incidence of grade ≥3 and grade 4 neutropenia in cycles 1–4, the mean duration of grade ≥3 neutropenia in cycles 2–4, the mean duration of grade 4 neutropenia in cycles 1–4, the incidence of FN (defined as body temperature ≥38.5 °C concurrent with ANC <1.0×109/L) in cycles 1–4.
The safety assessment was measured by reports of adverse events (AEs), changes in clinical laboratory values, vital signs and physical examinations.
Statistical analysis
This study was designed to show each of the mecapegfilgrastim arms is non-inferior to the filgrastim arm. The primary efficacy analyses were performed in the full analysis set (FAS). For duration of grade 3 or higher ANC decreases during cycle 1, we hypothesized the non-inferior margin as 1 day. Using an analysis of covariance (ANCOVA) model, the difference between patients treated with mecapegfilgrastim and patients treated with filgrastim would be calculated together with 95% confidence interval (CI). Non-inferiority would be established if the upper limit of the 95% CI was less than 1 day. If non-inferiority was established, the upper limit of the 95% CI could be further compared to 0 for assessment of superiority.
The sample size was calculated based on the primary end point of the duration of grade ≥3 ANC decrease at the first cycle of chemotherapy. Assuming a pre-specified non-inferiority margin of 1 day and a common standard deviation of 2 days, it was calculated that a total of 258 patients (86 per arm) were required to assess non-inferiority of mecapegfilgrastim (100 µg/kg or 6 mg) to the filgrastim at a one-sided significance level of 2.5% with 90% power. In order to allow for 20% of drop-outs and major protocol violations, a total of 330 patients were planned to be randomized with 110 patients in each arm. All statistical analysis was conducted using SAS 9.3 software.
Results
Baseline characteristics
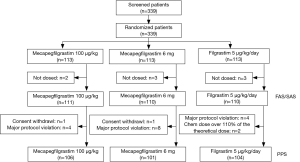
From March 2012 to November 2012, total of 339 patients from 22 centers in China were recruited into the study (Table S1). During the study, eight patients were excluded from the FAS, because they did not receive the treatment after randomization. At the end of cycle 1, total 331 (97.64%) patients received at least one dose of study drug and were included into the FAS. The per protocol set (PPS) included 311 patients, excluding 2 consent withdrawl, 16 major protocol violation, and 2 overdose chemotherapy treatment. The number of patients eligible for safety analysis set (SAS) was 331. Figure 1 illustrated the detailed patient disposition in each group in this study.
Full table
Demographic characteristics and disease status of patients were similar across treatment groups. Baseline vital signs, physical examination and general clinical characteristics were comparable among the three groups. Baseline ANC levels were within the normal range for all three groups and there were no statistically significant differences among the three groups at baseline (Table 1).

Full table
Primary efficacy endpoint
The efficacy analysis of FAS and PPS led to identical conclusions, only the results of FAS were reported here.
The mean duration of grade ≥3 neutropenia
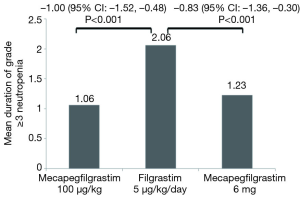
In cycle 1, the adjusted mean duration of grade ≥3 neutropenia was 1.06 (95% CI: 0.65, 1.26) days in mecapegfilgrastim 100 µg/kg group, 1.23 (95% CI: 0.84, 1.88) days in mecapegfilgrastim 6 mg group, and 2.06 (95% CI: 1.66, 2.46) days in the filgrastim group (Figure 2).
The mean difference between mecapegfilgrastim 100 µg/kg and filgrastim was –1.00 (95% CI: –1.52, –0.48), the mean difference between mecapegfilgrastim 6 mg and filgrastim was –0.83 (95% CI: –1.36, –0.30). (Figure 2). The upper bounds of 95% CI for the mean difference between mecapegfilgrastim and filgrastim were all <1 day (the predefined non-inferiority margin), the study met its primary endpoint.
Secondary endpoints
The incidence of grade ≥3 and grade 4 neutropenia in cycles 1–4
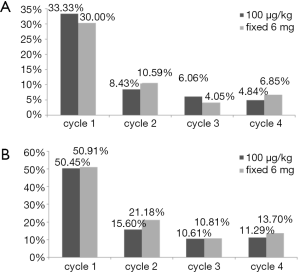
In cycle 1, there are 56 patients (50.45%) in mecapegfilgrastim 100 µg/kg arm, 56 patients (50.91%) in mecapegfilgrastim 6 mg arm, and 73 patients (66.36%) in filgrastim arm experienced grade ≥3 neutropenia. Compared with filgrastim arm, the incidence of grade ≥3 neutropenia was significantly lower in patient treated with mecapegfilgrastim 100 µg/kg (P=0.0147) and mecapegfilgrastim 6 mg (P=0.0064). There was no difference between the two mecapegfilgrastim arms in the incidence of grade ≥3 neutropenia (P=0.9470). There are 37 patients (33.33%) in mecapegfilgrastim 100 µg/kg arm, 33 patients (30.00%) in mecapegfilgrastim 6 mg arm, and 51 patients (46.36%) in filgrastim arm experienced grade 4 neutropenia. Compared with filgrastim, the incidence of grade 4 neutropenia was significantly lower in patient treated with mecapegfilgrastim 100 µg/kg (P=0.0454) and mecapegfilgrastim 6 mg (P=0.0036). There was no difference between the two mecapegfilgrastim arms in the incidence of grade 4 neutropenia (P=0.5688) (Table 2).

Full table
In cycle 2, 13 patients (15.66%) in mecapegfilgrastim 100 µg/kg arm and 18 patients (21.18%) in mecapegfilgrastim 6 mg arm experienced grade ≥3 neutropenia, there was no difference between these two groups (P=0.6917). There are seven patients (8.43%) in mecapegfilgrastim 100 µg/kg arm and nine patients (10.59%) in mecapegfilgrastim 6 mg arm experienced grade 4 neutropenia, there was no difference between these two groups (P=0.9469).
In cycle 3, the incidences of grade ≥3 neutropenia were 10.61% and 10.81% in mecapegfilgrastim 100 µg/kg and 6 mg groups. The incidences of grade 4 neutropenia were respectively 6.06% and 4.05% in these two groups. No difference was found between the two groups.
In cycle 4, the incidence of grade ≥3 neutropenia was 11.29% and 13.70% in mecapegfilgrastim 100 µg/kg and 6 mg groups. The incidence of grade 4 neutropenia was respectively 4.84% and 6.85% in these two groups. No difference was found between the two groups.
It was noted that there was a decreased trend in the incidence of grade ≥3 and grade 4 neutropenia as the treatment cycle increased (Figure 3).
The mean duration of grade ≥3 neutropenia in cycles 2–4
In cycle 2, the mean duration of grade ≥3 neutropenia was 0.36±0.99 days and 0.44±0.92 days in mecapegfilgrastim 100 µg/kg and 6 mg groups. In cycle 3, the mean duration of grade ≥3 neutropenia was 0.23±0.72 days and 0.26±0.79 days in mecapegfilgrastim 100 µg/kg and 6 mg groups. In cycle 4, the mean duration of grade ≥3 neutropenia was 0.63±2.39 days and 1.04±3.14 days in the two groups. No difference was found between the two groups.
The mean duration of grade 4 neutropenia in cycles 1–4
In cycle 1, the mean duration of grade 4 neutropenia was 0.61±0.96, 0.54±0.88, and 1.02±1.24 days in mecapegfilgrastim 100 µg/kg group, mecapegfilgrastim 6 mg group, and filgrastim group. The mean difference between mecapegfilgrastim 100 µg/kg and filgrastim groups was –0.40 (95% CI: –0.73, –0.08), the mean difference between mecapegfilgrastim 6 mg and filgrastim groups was –0.51 (95% CI: –0.84, –0.18), all the differences were statistically significant. The mean difference between two mecapegfilgrastim groups was 0.10 (95% CI: –0.22, 0.43) which was not statistically significant (Table 2).
In cycle 2, the mean duration of grade 4 neutropenia was 0.16±0.57 and 0.22±0.70 days in mecapegfilgrastim 100 µg/kg and 6 mg groups. In cycle 3, the mean duration of grade 4 neutropenia was 0.12±0.48 and 0.08±0.40 days. In cycle 4, the mean duration of grade 4 neutropenia was 0.08±0.38 and 0.53±2.35 days. All the differences between the two groups were not significant.
The incidence of FN
In cycle 1, there are 5 (4.50%), 0 (0%), and 2 (1.82%) patients experienced FN in mecapegfilgrastim 100 µg/kg group, mecapegfilgrastim 6 mg group and filgrastim group. There was no significant difference between the three groups (Table 2). During cycles 2 to 4, no FN was developed in patients in mecapegfilgrastim 100 µg/kg and mecapegfilgrastim 6 mg groups.
Subgroup analysis of primary endpoints
In order to determine whether the stratification factors (age, chemotherapy regimens) confounded the assessment of efficacy, an exploratory subgroup analyses according to the baseline body weight (≤70 vs. >70 kg) and chemotherapy (AC vs. AT) was performed for cycle 1.
For patients with body weight ≤70 kg, the mean duration of grade ≥3 neutropenia were respectively 1.17±1.28, 1.32±1.82 and 2.20±2.17 days, compared with filgrastim group, the mean duration of grade ≥3 neutropenia were significantly shorter in patients with mecapegfilgrastim 100 µg/kg and 6 mg treatment, the difference were respectively –0.98 (95% CI: –1.56, –0.39) and –0.91 (95% CI: –1.51, –0.31). There is no difference between two mecapegfilgrastim arms. For patients with body weight >70 kg, no difference was found in the mean duration of grade ≥3 neutropenia between the three groups (Table 3).
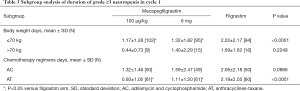
Full table
For patients treated with AT chemotherapy, the mean duration of grade ≥3 neutropenia respectively 0.93±1.05, 1.11±1.20 and 2.18±2.05 days, compared with filgrastim group, the mean duration of grade ≥3 neutropenia were significantly shorter in patients with mecapegfilgrastim two dose regimens treatment, the difference were respectively –1.20 (95% CI: –1.81, –0.59) and –1.10 (95% CI: –1.71, –0.49). For patients treated with AC chemotherapy, there was no significant difference between the three groups (Table 3).
Safety analysis
In cycle 1, total 320 patients experienced AEs in mecapegfilgrastim 100 µg/kg arm (105 patients, 94.59%), mecapegfilgrastim 6 mg arm (107 patients, 97.27%) and filgrastim arm (108 patients, 98.18%). Total seven patients reported serious adverse events (SAEs) in mecapegfilgrastim 100 µg/kg (three patients), mecapegfilgrastim 6 mg (one patient) and filgrastim (three patients) arms. All of the SAEs were considered not related to investigational treatment by the investigators. No unexpected AE was observed. The most frequently reported AEs possibly related to treatment were shown in Table 4. Grade 3 AEs were reported in mecapegfilgrastim 100 µg/kg group (two patients), 6 mg fixed dose group (three patients) and filgrastim group (two patients). Overall, no significant difference was detected among the three groups in terms of the incidence of all AEs, the treatment related AEs, and the SAEs.
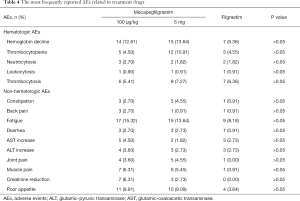
Full table
Discussion
Neutropenia is the main dose-limiting toxicity of chemotherapy, always leading to dosage adjustment or treatment interruption, which could compromise the efficacy, so it is very important to ensure sufficient treatment cycles and dosage for chemotherapy. In a phase II trial, mecapegfilgrastim demonstrated better clinical efficacy and similar safety profile as the secondary prophylactic therapy for neutropenia compared with filgrastim in breast cancer patients (13).
However, in above phase II trial, the evaluation of the efficacy and safety of mecapegfilgrastim was mainly focused on the secondary prophylactic therapy in one treatment cycle. This phase III trial was designed to further investigate the efficacy and safety of mecapegfilgrastim as the primary prophylactic therapy in four consecutive treatment cycles, and also to explore whether the fixed 6 mg dosage showed a similar safety and efficacy to the weight-based dose of 100 µg/kg. The results of this study showed that the single dose of mecapegfilgrastim (at either 100 µg/kg or 6 mg) was as safe and effective as daily injections of filgrastim as the primary prophylactic therapy in the first chemotherapy cycle in breast cancer patients, which was consistent with the results of some other phase II and phase III studies in breast cancer or other malignant tumors.
DSN was considered as a sensitive endpoint in assessing biosimilarity of filgrastim in (neo) adjuvant breast cancer, any variations in DSN can be considered as a direct consequence of differences between activity of reference and biosimilar filgrastim (12). Moreover, risk of infection is directly proportional to severity and duration of neutropenia, making DSN a clinically relevant endpoint (14,15). As for the primary endpoint of this study, the mean duration of grade ≥3 neutropenia in both mecapegfilgrastim groups were non-inferior to that in the filgrastim group in cycle one. The mean difference of the duration of grade ≥3 neutropenia between mecapegfilgrastim 100 µg/kg and filgrastim was –1.00 day (95% CI: –1.52, –0.48 day), the difference between mecapegfilgrastim 6 mg and filgrastim was –0.83 day (95% CI: –1.36, –0.30 day). The upper bounds of 95% CI for the mean difference between mecapegfilgrastim and filgrastim were all within the predefined margin of 1 day, which indicated that the efficacy of mecapegfilgrastim in reducing duration of grade ≥3 neutropenia was non-inferior to filgrastim.
Furthermore, the upper bounds of 95% CI for the mean difference between mecapegfilgrastim and filgrastim were all <0, which indicated that compared with filgrastim, mecapegfilgrastim at dosage of either 100 µg/kg or 6 mg might be superior in reducing duration of grade ≥3 neutropenia. The superiority might be the result of the longer half-life time of 55.99 hours and the unique linking structure of mecapegfilgrastim. For the duration of grade 4 neutropenia, compared with filgrastim, the duration of grade 4 neutropenia were significantly shorter in patients treated with mecapegfilgrastim 100 µg/kg and the fixed dosage of 6 mg. The reduced DSN could be associated with decreased risk of infection and shorter period of hospitalization, which could save and make good use of the limited medical resources to serve more patients, and make the patients spend more time with their family at home.
In this study, for some secondary endpoints (the incidence of grade ≥3 and grade 4 neutropenia, the mean duration of grade 4 neutropenia), there were significant differences between two dosage groups of mecapegfilgrastim and filgrastim, indicating that in some parameters, mecapegfilgrastim may be better than filgrastim in supporting cytotoxic chemotherapy. These results were consistent with the results from phase III study of the mecapegfilgrastim in NSCLC, which showed that for the duration of grade 4 neutropenia in cycles 2 to 4 and for the incidence of FN, the differences between mecapegfilgrastim and filgrastim were significant (11). And the phase II trial of mecapegfilgrastim in breast cancer also showed similar results (13). A systematic review also revealed similar results, showing better efficacy and effectiveness for pegfilgrastim than filgrastim (16). All these results suggest that longer-acting rhG-CSF might provide additional clinical benefit, the mechanism for such findings was unclear.
In our study, we further evaluated the efficacy of mecapegfilgrastim in consecutive-cycle applications, which showed a declining trend of the incidence of grade ≥3 and grade 4 neutropenia along with the treatment cycles increased (Figure 2), which indicated that patients may benefit more from consecutive-cycle treatment of mecapegfilgrastim (at either 100 µg/kg or 6 mg) in prevention of severe neutropenia. This hypothesis would be further evaluated in the well-designed clinical trials.
In clinical practice, a fixed-dose regimen would begenerally preferred for administration. However, there were concerns about the fixed dosage for the lack of efficacy in over-weighted patients and occurrence of sever AEs in less-weighted patients. So, in this study, we added a fixed-dose group of mecapegfilgrastim at 6 mg, and compared the efficacy of the two dosage regimens of mecapegfilgrastim (100 µg/kg and a fixed dosage of 6 mg) in different-weight subgroups in all 4 cycles. The results showed, there was no significant difference between the fixed 6 mg and 100 µg/kg of mecapegfilgrastim in duration of grade ≥3 neutropenia, incidence of grade ≥3 neutropenia and incidence of bone pain during all cycles. The previous studies also demonstrated that mecapegfilgrastim fixed 6 mg or 100 µg/kg dosage provided comparable benefit as filgrastim (2,11). Thus, the efficacy and safety of the fixed 6 mg-dose regimen is appropriate for patients, and should be recommended in the clinical practice in terms of the convenience of administration.
In China, AT and AC chemotherapy regimens were commonly used in clinical practice, and were also recommended in Chinese treatment guidelines for breast cancer patients. These two regimens have proven dose limiting hematological toxicity with grade 3–4 neutropenia. In this study, for AT regimen, patients treated with mecapegfilgrastim experienced shorter duration of grade ≥3 neutropenia compared with filgrastim. For patients treated with AC regimen, mecapegfilgrastim exhibited comparable efficacy with filgrastim for the duration of grade ≥3 neutropenia. These results indicated that patients treated with mecapegfilgrastim might benefit more for neutropenia prophylaxis when they treated with AT chemotherapy, which possessed stronger myelosuppression than AC chemotherapy.
For safety profile, there was no significant difference between mecapegfilgrastim (either 100 µg/kg or fixed 6 mg) and filgrastim in terms of the incidence of all AEs, including the common events of pain and decreased hemoglobin. In this phase III study, no unexpected AEs, fixed-dosage related AEs, nor consecutive-cycle related AEs were found. All of the SAEs were considered not related to the investigational treatment by the investigators. Patients in megapegfilgrastim groups were well tolerated.
There is also a limitation in this study. We only included the breast cancer patients in AT/AC chemotherapy regimens limited by the rules of randomized-control-study design. In the future, we plan to evaluate the effectiveness and safety of mecapegfilgrastim further in the real-world study, in which more practical chemotherapy regimens would be included.
In conclusion, this study demonstrated that long-acting mecapegfilgrastim (100 µg/kg and fixed 6 mg) is very effective and well tolerated when administered in the primary prophylaxis of chemotherapy induced neutropenia and in consecutive-cycle treatment. In some clinical parameters, mecafilgrastim is non-inferior and even superior to filgrastim. The fixed 6 mg-dose regimen showed similar efficacy and safety profile compared with 100 µg/kg regimen, and would be the preference in clinical practice, due to the convenient once-per-cycle administration and high-degree treatment compliance for the patients. This study provided new evidence for the novel long-acting rhG-CSF, mecapegfilgrastim, which would be a new alternative for clinical practice for prophylaxis of chemotherapy induced neutropenia.
Acknowledgments
The authors acknowledge the contribution of: Huang Zhongcheng (Department of General Surgery, Hunan Provincial Hospital, Changde, China); Liu Yunpeng (Department of Oncology, The First Affiliated Hospital, China Medical University, Shenyang, China); Wu Youhua (Department of Oncology, The First Hospital University of South China, Hengyang, China); Li Xinzheng (Department of Breast Surgery, Shanxi Cancer Hospital, Taiyuan, China); Zhang Tao (Department of Oncology, Chengdu Military General Hospital, Chengdu, China); Wang Ningju (Department of Oncology, Affiliated Hospital of Ninxia Medical University, Yinchuan, China); Gao Jianfei (Department of Oncology, Wuhan General Hospital, Guangzhou Command of PLA, Wuhan, China); Sun Heqing (Department of Breast Surgery, Yangzhou First People’s Hospital, Yangzhou, China); Huang Feizhou (Department of General Surgery, The Third Xiangya Hospital Of Central-South University, Changsha, China); Dang Chengxue (Department of Tumor Surgery, The First Affiliated Hospital, Medical school of Xi’an Jiaotong University, Xi’an, China); Ding Kefeng (Department of Oncology, The Second Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China); Liu Yunjiang (Department of Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China) for their contributions to the enrollment of trial subjects. Cunnan Dong assisted with the writing of this manuscript. This study was funded by Hengrui Medicine Co., Ltd. (Lianyungang, China).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethics Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the medical center (2011-11-102), and the protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients or their guardians signed the informed consent before enrollment. This trial was approved by the National Medical Products Administration of China (registration number: 2010L00501) and registered on ClinicalTrials.gov (NCT01611051).
References
- Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302-13. [Crossref] [PubMed]
- Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol 2003;14:29-35. [Crossref] [PubMed]
- Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol 2002;20:727-31. [Crossref] [PubMed]
- Nasti G, Talamini R, Antinori A, et al. AIDS-related Kaposi’s sarcoma: evaluation of potential new prognostic factors and assessment of the AIDS clinical trial group staging system in the HAART era—the Italian cooperative group on AIDS and tumors and the Italian cohort of patients naive from antiretrovirals. J Clin Oncol 2003;21:2876-82. [Crossref] [PubMed]
- Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol 2005;23:1178-84. [Crossref] [PubMed]
- Biganzoli L, Untch M, Skacel T, et al. Neulasta (pegfilgrastim): a once-per-cycle option for the management of chemotherapy-induced neutropenia. Semin Oncol 2004;31:27-34. [Crossref] [PubMed]
- Molineux G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr Pharm Des 2004;10:1235-44. [Crossref] [PubMed]
- Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer 2011;11:404. [Crossref] [PubMed]
- Weycker D, Malin J, Barron R, et al. Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in patients with cancer receiving chemotherapy. Am J Clin Oncol 2012;35:267-74. [Crossref] [PubMed]
- Yan B, Zhang W, Lu F, et al. Safety of polyethylene glycol recombinant human granulocyte colony-stimulating factor in treating non-small cell lung cancer patients at I b stage. Asian Pac J Trop Med 2013;6:912-5. [Crossref] [PubMed]
- Zhou C, Huang Y, Wang D, et al. A randomized multicenter phase III study of single administration of mecapegfilgrastim (HHPG-19K), a pegfilgrastim biosimilar, for prophylaxis of chemotherapy-induced neutropenia in patients with advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer 2016;17:119-27. [Crossref] [PubMed]
- Krendyukov A, Schiestl M, Höbel N, et al. Clinical equivalence with G-CSF biosimilars: methodologic approach in a (neo) adjuvant setting in non-metastatic breast cancer. Support Care Cancer 2018;26:33-40. [Crossref] [PubMed]
- Wang T, Wu B, Hu X, et al. A randomized multicenter phase II trial of mecapegfilgrastim single administration versus granulocyte colony-stimulating growth factor on treating chemotherapy-induced neutropenia in breast cancer patients. Ann Transl Med 2019;7:196. [Crossref] [PubMed]
- Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 1966;64:328-40. [Crossref] [PubMed]
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004;100:228-37. [Crossref] [PubMed]
- Pfeil AM, Allcott K, Pettengell R, et al. Efficacy, effectiveness and safety of long-acting granulocyte colony-stimulating factors for prophylaxis of chemotherapy-induced neutropenia in patients with cancer: a systematic review. Support Care Cancer 2015;23:525-45. [Crossref] [PubMed]