
The expression and clinical significance of serum IL-17 in patients with primary biliary cirrhosis
Introduction
Primary biliary cirrhosis (PBC) is one of the autoimmune liver diseases. Due to the progressive destruction of the bile ducts in the intrahepatic lobes, the microenvironment of the bile is damaged and the toxic bile components are retained in the cells. Long-term intrahepatic cholestasis can lead to liver fibrosis and cirrhosis, and ultimately liver failure (1-3). In recent years, the incidence of PBC is on the rise in China (4,5), and the mortality rate is very high, so early screening and treatment are very important (6,7). Th17 cell is a new independent subtype of CD4+ T cells different from Th1, Th2 and Tregs. It is characterized by the high secretory cytokine IL-17 and plays an important role in host defense, mediating inflammation infection and autoimmune diseases, etc. (8-10). IL-17 is mainly a pro-inflammatory factor secreted by Th17 cells after stimulation. It induces tissue inflammation by inducing many pro-inflammatory cytokines and chemokines (11), in addition to profibrotic effects, Helps organ fibrosis (12,13). However, the relationship between Th17/CD4+ T and related cytokines and the pathogenesis of PBC is not yet clear. This study retrospectively analyzed the clinical features and laboratory parameters of patients with PBC combined with the expression of IL-17 and related cytokines and accumulated experience for clinical treatment of patients, providing reference value and scientific basis.
Methods
Study subjects
From January 2015 to December 2015, a total of 127 patients with PBC were enrolled in the Department of Hepatology and Infectious Diseases at the First Affiliated Hospital of Xinjiang Medical University, including 16 males (12.60%) and 111 females (87.40%). The ratio of male to female is 1:6.94, age 32–76 years old. According to the Diagnostic Criteria of the American Society of Liver Diseases in 2009 and the Diagnostic Criteria of the European Association of Liver Diseases (14), the diagnosis can be determined by having two of the following three items: (I) serum anti-mitochondrial antibody (AMA) positive; (II) elevated alkaline phosphatase (ALP) and/or glutamyl transpeptidase (GGT) levels; (III) typical pathological changes in the liver. Consequently, 100 healthy people were selected as control group. Patients volunteered to participate in the tests and signed informed consent.
Exclusion criteria: (I) occupying or obstructing the intrahepatic and extrahepatic bile ducts; (II) viral hepatitis, alcoholic hepatitis, drug-induced liver injury and hepatocellular carcinoma; (III) recently suffering from serious infectious diseases, cardiovascular diseases; (IV) receiving Immunosuppressive drug or enhancer drug therapy; (V) autoimmune hepatitis (AIH) primary sclerosing cholangitis (PSC) and autoimmune liver disease overlap syndrome.
Instruments and reagents
AU5800 automatic biochemical analyzer (Beckman Coulter, USA), FACSCalibur type flow cytometer (BD company, USA), Clinical Biochemical System Reagent (Beckman Coulter, USA), BD inflammatory factor test kit (BD company, USA), Rabbit anti human IL-17 antibody (Wuhan Dr. Biological Engineering Co., Ltd.). The biotin-labeled universal secondary antibody kit was purchased from Beijing Zhongshan Biotechnology Co., Ltd. Maxima SYBR Green/ROX qPCR Master Mix, RevertAidTM First strand cDNA Synthesis Kit (United States Thermo Fisher scientific company), Trizol (Invitrogen, USA) and so on.
Observation indicators
General conditions
Patient’s gender, age, body mass index and clinical symptoms.
Biochemical indicators
Patient serum detection of alanine aminotransferase (ALT), aspartate aminotransferase (AST), GGT (rate method), ALP (PNP colorimetric method) and so on.
Autoantibodies
AMA, antinuclear antibody (ANA), and anti-flat muscle antibody (SMA) were detected using an autoantibody profile IgG detection kit (indirect immunofluorescence) produced by Oumen Medical Laboratory Diagnostics Co., Ltd. Anti-mitochondrial antibody M2 (AMA-M2), anti-Sjogren’s syndrome A antibody (SSA) and anti-Sjogren’s syndrome B antibody (SSB) were detected with the IgG antibody detection kit (Western blot) produced by Omega Medical Experimental Diagnosis Co, Ltd.
ANA karyotype
The fluorescence staining intensity and fluorescence pattern of ANA were observed under fluorescence microscope. Homogeneous type: The nuclei of interphase cells showed uniform fluorescence, and the chromosome fluorescence of mitotic cells concentrated increased, and the chromosome fluorescence of mitotic cells concentrated increased. Granular type: intermittent nucleus fluorescence was granular, and mitotic chromosome fluorescence was not detected; cytoplasmic pattern: coarse granule-like fluorescein in the cytoplasm; Centromere pattern: punctate fluorescence with the same size and even distribution in the nucleus of the interphase, and concentrated punctate fluorescence in centralized chromosomes of mitotic cells. Nuclear membrane type: Interphase nuclei showed uniform fluorescence, perinuclear enhancement, and chromosome negative in mitotic cells; nucleolar type: interphase nucleoli is negative, and the dividing cells are negative for chromosome concentration. Spotted type: particle-like fluorescence appears in the nucleus.
Flow cytoplasmic multiple protein quantification (CBA)
The standard was diluted to different concentrations according to the requirements of the kit, and a mixture of five cytokines (TNF-α, IFN-γ, IL-6, IL-10 and IL-17) antibodies was added to each tube to be tested. 50 µL of beads, then add 50 µL of different concentration standards or serum samples to be tested, then add 50 µL of PE-labeled detection antibody, mix well, incubate at room temperature for 2 h in the dark, wash with 1 mL of washing solution (3 000 r/min, centrifuge for 5 min, discard After the supernatant, the cells were resuspended by adding 300 µL of buffer, and the expression of cytokines was detected immediately after 3–5 minutes.
Real-time fluorescent quantitative PCR
Total RNA extraction
The total RNA of whole blood mononuclear cells was extracted by Trizol reagent and carried out according to the instructions.
Reverse transcription
The total volume of RT reaction is 20 µL, according to the instructions of the reverse transcription kit.
Primer design
Primer sequence according to the known sequence on GenBank, see Table 1.

Full table
Real-time quantitative PCR
2 µL of cDNA template, 10 µL of SYBR Green 1 Mix dye, 0.5 µL of upstream and downstream primers, 7 µL of ddH2O, and 20 µL of system. The following are the cycling conditions: 55 °C for 2 minutes, one cycle; 95 °C for 10 minutes, one cycle; 95 °C denaturation for 15 seconds, 60 °C annealing for 60 seconds, 40 cycles; detection of fluorescence signals and plot the dissolution curve. RNA expression analysis used the difference between the measured value and the internal reference CT value as the relative expression of the factors to be measured.
HE staining method
After paraffin section dewaxing and dehydration, hematoxylin staining for 8 minutes, washing three times, ethanol hydrochloride differentiation for 1 second, washing once, PBS returned to blue for 5 min, eosin staining for 3 minutes, washing three times, gradient ethanol dehydration, xylene transparent, air drying and neutral Gum seals. Observe liver morphological changes.
Immunohistochemical method
After paraffin section dewaxing and hydration, PBS disposed fresh 3% hydrogen peroxide to remove endogenous peroxidase, citric acid microwave oven heated for 10 minutes to repair antigen, cooled to room temperature, and sheep serum was added for non-specific site closure, incubated at 37 °C incubator for 30 minutes, dripped with an antibody (1:150), put in 4 °C refrigerator overnight. The next day, sheep anti-rabbit IgG labeled with HRP was dripped and incubated at 37 °C for 25 minutes. The staining was observed under microscope. If there was positive staining, tap water stopped staining. After hematoxylin re-staining, hydrochloric acid ethanol differentiation, PBS returned to blue. After dehydration and transparency, the neutral gum seals were made. The test was performed with a section positively stained as a positive control, and a primary antibody was replaced with PBS as a negative control.
Statistical methods
Statistical analysis was performed using SPSS16.0 statistical software, and the normal distribution of measurement data was expressed by S. The normal measurement data were compared between the two groups by independent sample t-test analysis and the non-normal measurement data were compared using the Mann-Whitney U test. The count data was analyzed by χ2 and correlation coefficient analysis was performed using correlation analysis. P<0.05 was considered statistically significant.
Results
Baseline information and clinical features of PBC patients
As can be seen from Table 2, fatigue and jaundice were the most common symptoms in 127 patients, 37.8% and 32.28% respectively, followed by skin itching, poor appetite and weight loss.
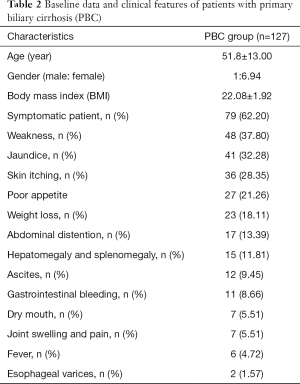
Full table
Biochemical indicators of PBC group and healthy control group
This study examined the clinical biochemical parameters of serum AST, ALT, ALP and GGT in PBC group and healthy control group, as shown in Table 3. The results showed that compared with control group, PBC group ALT, AST, ALP, GGT, TBIL, ALB The 5'-NT level were significantly increased (P<0.01), and the differences were statistically significant.
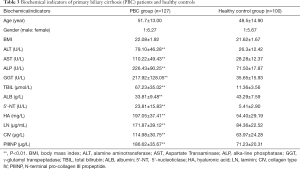
Full table
Expression of autoantibodies in patients with PBC
This study examined autoantibodies such as ANA, AMA, AMA-M2, SMA, SSA and SSB in the PBC group and healthy controls, and tested the ANA titer, as shown in Tables 4,5. The results showed that the positive rates of ANA, AMA, AMA-M2, SMA and SSB in the PBC group were higher than those in the control group (P<0.05), and the differences were statistically significant. The main karyotype in ANA is granular and homogenization and cytoplasmic forms also account for a certain proportion.

Full table
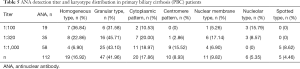
Full table
The titer is dominated by medium to high titers (≥1:320).
The expression level of serum related cytokines in PBC group and healthy control group
The expression of cytokines in peripheral blood of patients with PBC was detected by CBA. The results are shown in Table 6 shows that the levels of TNF-α, IL-6 and IL-17 are significantly higher than those of healthy controls, and the difference is statistically significant (P<0.01), IFN-γ and IL-10 also had a slight increase, the differences were statistically significant (P<0.05).
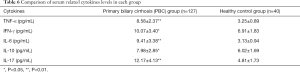
Full table
Correlation between IL-17 and biochemical indicators in PBC group
The correlation between cytokine IL-17 and clinical biochemical indicators is shown in Table 7. The results showed that IL-17 was positively correlated with ALT, ALP, GGT and CIV (r=0.350, P=0.013; r=0.373, P=0.008; r=0.337, P=0.017; r=0.349, P=0.021).
Full table
IL-17 mRNA expression level in patients with PBC
Detection of IL-17 mRNA expression in lymphocytes of 127 patients in PBC group and 40 control group. As shown in Table 8, the expression of IL-17 mRNA in peripheral blood lymphocytes of control group was (0.89±0.30), compared with the PBC group, The expression of IL-17 mRNA in peripheral blood lymphocytes of PBC group was significantly increased (1.34±0.58), and the difference was statistically significant (P<0.01).
Full table
Liver tissue morphology in healthy people and PBC patients
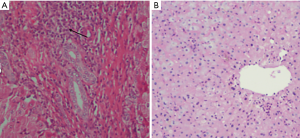
HE staining of liver tissue in patients with PBC (Figure 1A) showed hepatocyte swelling, a few scattered focal necrosis, cytoplasmic loosening, enlargement of the portal area, and a large number of lymphocytes and neutrophil infiltration, bile duct epithelial degeneration and bile duct pericellular epithelium A granuloma formation. HE staining of healthy human liver tissue (Figure 1B) showed that the nucleus of hepatocytes is large and round, centered, with rich chromatin and normal morphology. Hepatic lobule structure rules. The hepatocytes are arranged radially with the central vein as the axis. There is no inflammatory cell infiltration in the lobules and portal area.
Immunohistochemistry results of IL-17 in the liver of patients with PBC
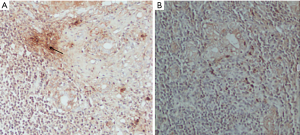
As shown in Figure 2A,B, there were almost no positive cells in normal liver tissue, and IL-17 positive cells appeared in PBC liver tissue, which was brownish and mainly concentrated in the bile duct. The results showed that IL-17 positive cells in portal area of PBC patients (n=20) were significantly higher than those in healthy control group (n=4) (P<0.01) (Table 9).

Full table
Discussion
The pathogenesis of PBC is still unknown (15), which may destroy autoimmune tolerance under the influence of external factors (16), genetic factors (17) immune regulatory system (18), and autoimmune response mediates the result of persistent hepatocyte damage. The end result was cell damage. In this study, the main clinical manifestations of PBC patients were fatigue, jaundice, pruritus, anorexia, and weight loss, consistent with the literature (19-21). This may be due to abnormal adrenaline secretion caused by central nervous system disorders and cholestasis caused by bile salt stimulation of skin nerve endings. The positive rates of autoantibodies ANA, AMA, AMA-M2, SMA and SSB in this study were higher than those in the control group, which was consistent with the study by Granito et al. (22) and Sun et al. (23). The ANA karyotype was mainly granular, homogenous and cytoplasmic. It plays an auxiliary diagnostic role in PBC screening.
In the laboratory index test, cholestasis is the main cause. Ali et al. (21) study shows, bile acid can dialyse the lipomembrane of ALP from the lipid membrane under the action of surface activity, which promoted the increase and release of hepatogenic ALP into the blood. Clastic necrosis around the portal area was also positively correlated with ALP level. The index that is significantly increased in synchronization with this is GGT, which is up to 8 times higher than the normal reference value. It may increase the intrahepatic and intrahepatic biliary tract pressure due to biliary obstruction and hepatocyte swelling promotes the production of a large amount of GGT. Elevated levels of bilirubin are important indicators of disease progression in patients with PBC, reflecting the extent of bile duct destruction and cholestasis. This study showed that the levels of ALT, AST, ALP, GGT, TBIL, ALB, and 5'-NT in PBC patients were significantly increased, consistent with the literature (24), showing that serum 5'-NT levels were parallel with GGT, which was consistent with the clinical value of this index in the diagnosis of hepatobiliary diseases.
In autoimmune diseases, immune effector factors form complex cytokine networks through mutual antagonism or self-promotion to regulate immune response. IL-17 is a specific cytokine secreted by Th17 cells. It plays an important role in autoimmune diseases by promoting the secretion, release and chemotaxis of various inflammatory factors to the site of inflammation and synergizing with them to amplify biological effects (25,26). Recently, some scientists have established arthritis animal models and found that treatment with IL-17 antagonist has significant effects in delaying disease progression in both early and late stages (27). Studies have found that a large number of Th17 cells infiltrate in the liver tissue of patients with PBC; co-culture of spleen CD4+ T cells and hepatocytes in normal mice can secrete more IL-17 than spleen CD4+ T cells alone, suggesting that Th17 cells participate in the liver autoimmune inflammation process in the hepatocyte microenvironment (28). This study found that peripheral blood IL-17 levels in patients with PBC were significantly higher than those in normal subjects, consistent with the literature (13,29-31), suggesting that elevated cytokine IL-17 may be due to hepatocytes in the microenvironment of inflammatory injury. It is easy to induce the differentiation and release of Th17 cells and reaches the liver through blood circulation which mediates the development of autoimmune response. Simultaneously, it secretes a large number of inflammatory factors to promote liver inflammatory reaction. On the other hand, IL-17 can promote the proliferation of autoreactive B cells and the production of autoantibodies (32).
IL-17 was found to be positively correlated with ALT, ALP and GGT in the detection of clinical indicators. ALT is a sensitive indicator of hepatocyte damage, suggesting that IL-17 is involved in the immune process that damages liver cells. ALP and GGT are the key indicators in the course of PBC, which can reflect the inflammation and deposition in the range of liver injury. GGT is also significantly associated with liver tissue inflammation, reflecting the severity of liver pathological damage (33), speculation IL-17 is closely related to the progression of PBC disease and may have a certain effect on lymphocyte inflammatory infiltration. Corresponding to the pathological results, inflammatory cells accumulate around the bile duct and promote apoptosis of bile duct epithelial cells, which can accelerate the process of disease.
This experiment examined the expression levels of IL-17-related cytokines. TNF-α and IL-6 are T cells secreting inflammatory factors, which can aggravate the process of inflammatory reaction (34). IL-10 is an anti-inflammatory factor, which inhibits the activation and adhesion of inflammatory cells, reduces the synthesis and release of inflammatory factors, and reduces the inflammatory response, thereby reducing liver injury (35). In this study, the level of IL-10 in PBC patients was higher than that in healthy controls. This may be due to the fact that there is no period in this group; the early patients may have a compensatory increase of IL-10 to protect the immune balance. IFN-γ is involved in the activation of T lymphocytes, which suggests that T lymphocytes in PBC patients are activated and cytokines are activated to maintain the differentiation and development of Th17 cells. The results showed that the levels of serum TNF-α, IFN-γ, IL-6 and IL-10 were significantly increased in PBC group, indicating that IL-17 was involved in the pathogenesis of PBC by regulating pro-inflammatory cytokines. IL-6 is a cytokine secreted mainly by APCs. It is one of the initiating factors of TGF-β differentiation. In the case of coexistence of IL-6 and TGF-β, IL-6 can induce a large number of Th17 cells to proliferate, and then mature Th17 cells can secrete IL-6 (36,37). The results of this study are consistent with the literature (38-40). IL-6 production can regulate Th17 cells in the differentiation stage and promote inflammatory response.
In addition, the expression of IL-17 mRNA in peripheral blood mononuclear cells of PBC group was significantly higher than that of healthy control group. The expression of IL-17 gene showed an upward trend in PBC disease. It was speculated that there might be an increased expression of activated Th17 and related cytokines in PBC patients. This study supports that IL-17 cytokines play an important role in the pathogenesis of PBC.
The purpose of this study is to explore the role of IL-17 in PBC. However, there are several inadequacies that should be noted. Firstly, there is no pathological staging of PBC disease. Secondly, the exact mechanism of IL-17 signaling pathway in PBC remains to be further explored. In summary, IL-17 is highly expressed in PBC disease, and higher IL-17 is associated with PBC, suggesting that IL-17 may be involved in the process of inflammation and injury of liver tissue. IL-17 plays a key role in promoting cellular and humoral immune responses. It is speculated that IL-17 may become a potential therapeutic intervention focus as a new direction of targeted therapy for PBC diseases.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (81760372).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the medical ethics committee of First Affiliated Hospital of Xinjiang Medical University (20150115-01). Patients volunteered to participate in the tests and signed informed consent.
References
- Namisaki T, Moriya K, Noguchi R, et al. Liver fibrosis progression predicts survival in patients with primary biliary cirrhosis. Hepatol Res 2017;47:E178-86. [Crossref] [PubMed]
- Invernizzi P, Selmi C, Gershwin ME. Update on primary biliary cirrhosis. Dig Liver Dis 2010;42:401-8. [Crossref] [PubMed]
- Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol 2014;60:210-23. [Crossref] [PubMed]
- Qin B, Liang Y, Yang Z, et al. Scientific Publications on Primary Biliary Cirrhosis from 2000 through 2010: An 11-Year Survey of the Literature. PLoS One 2012;7:e35366. [Crossref] [PubMed]
- Zhang XX, Wang LF, Jin L, et al. Primary biliary cirrhosis-associated hepatocellular carcinoma in Chinese patients: incidence and risk factors. World J Gastroenterol 2015;21:3554-63. [Crossref] [PubMed]
- Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: A systematic review. J Hepatol 2012;56:1181-8. [Crossref] [PubMed]
- Berg PA. The role of the innate immune recognition system in the pathogenesis of primary biliary cirrhosis: a conceptual view. Liver Int 2011;31:920-31. [Crossref] [PubMed]
- Isono F, Fujita-Sato S, Ito S. Inhibiting RORγt/Th17 axis for autoimmune disorders. Drug Discov Today 2014;19:1205-11. [Crossref] [PubMed]
- Kim BS, Park YJ, Chung Y. Targeting IL-17 in autoimmunity and inflammation. Arch Pharm Res 2016;39:1537-47. [Crossref] [PubMed]
- Beringer A, Noack M, Miossec P. IL-17 in Chronic Inflammation: From Discovery to Targeting. Trends Mol Med 2016;22:230-41. [Crossref] [PubMed]
- Zhang H, Bernuzzi F, Lleo A, et al. Therapeutic Potential of IL-17-Mediated Signaling Pathway in Autoimmune Liver Diseases. Mediators Inflamm 2015;2015:436450. [Crossref] [PubMed]
- Gao B, Waisman A. Th17 cells regulate liver fibrosis by targeting multiple cell types: many birds with one stone. Gastroenterology 2012;143:536-9. [Crossref] [PubMed]
- Shi T, Zhang T, Zhang LN, et al. The Distribution and the Fibrotic Role of Elevated Inflammatory Th17 Cells in Patients With Primary Biliary Cirrhosis. Medicine 2015;94:e1888. [Crossref] [PubMed]
- Lindor KD, Gershwin ME, Poupon R, et al. Primary biliary cirrhosis. Hepatology 2009;50:291-308. [Crossref] [PubMed]
- Liang DY, Hou Y, Luo L. Altered expression of miR-92a correlates with Th17 cell frequency in patients with primary biliary cirrhosis. Int J Mol Med 2016;38:131-8. [Crossref] [PubMed]
- Juran BD, Lazaridis KN. Environmental factors in primary biliary cirrhosis. Semin Liver Dis 2014;34:265-72. [Crossref] [PubMed]
- Selmi C, Diana A, Cocchi CA, et al. Environmental factors and the induction of autoimmunity in primary biliary cirrhosis. Expert Rev Clin Immunol 2008;4:239-45. [Crossref] [PubMed]
- Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of live: the UK-PBC national study. Hepatology 2013;58:273-83. [Crossref] [PubMed]
- Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015;386:1565-75. [Crossref] [PubMed]
- Hegade VS, Bolier R, Elferink RP, et al. A systematic approach to the management of cholestatic pruritus in primarybiliary cirrhosis. Frontline Gastroenterol 2016;7:158-66. [Crossref] [PubMed]
- Ali AH, Carey EJ, Lindor KD. Diagnosis and management of primary biliary cirrhosis. Expert Rev Clin Immunol 2014;10:1667-78. [Crossref] [PubMed]
- Granito A, Muratori P, Quarneti C, et al. Antinuclear antibodies as ancillary markers in primary biliary cirrhosis. Expert Rev Mol Diagn 2012;12:65-74. [Crossref] [PubMed]
- Sun L, Wang Y, Liu Y, et al. Differential characteristics of AMA-M2 autoantibody in primary biliary cirrhosis and non-PBC patients. Zhonghua Gan Zang Bing Za Zhi 2015;23:343-9. [PubMed]
- Qian C, Jiang T, Zhang W, et al. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine 2013;64:172-80. [Crossref] [PubMed]
- Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012;11:763-76. [Crossref] [PubMed]
- Huang J, Yuan Q, Zhu H, et al. IL-17C/IL-17RE Augments T Cell Function in Autoimmune Hepatitis. J Immunol 2017;198:669-80. [Crossref] [PubMed]
- Church LD, Filer AD, Hidalgo E, et al. Rheumatoid synovial fluid interleukin-17-producing CD4T cells have abundant tumor necrosis factor-alpha co-expression, but little interleukin-22 and interleukin-23R expression. Arthritis Res Ther 2010;12:R184-96. [Crossref] [PubMed]
- Lan RY, Salunga TL, Tsuneyama K, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun 2009;32:43-51. [Crossref] [PubMed]
- Qian C, Jiang T, Zhang W, et al. Increased IL-23 and IL-17 expression by peripheral blood cells of patients with primary biliary cirrhosis. Cytokine 2013;64:172-80. [Crossref] [PubMed]
- Ge J, Wang K, Meng QH, et al. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 2010;30:60-7. [Crossref] [PubMed]
- Yang CY, Ma X, Tsuneyama K, et al. IL-12/Th1 and IL-23/Th17 Biliary Microenvironment in Primary Biliary Cirrhosis: Implications for Therapy. Hepatology 2014;59:1944-53. [Crossref] [PubMed]
- Wasilewska A, Winiarska M, Olszewska M, et al. Interleukin-17 inhibitors. A new era in treatment of psoriasis and other skin diseases. Postepy Dermatol Alergol 2016;33:247-52. [Crossref] [PubMed]
- Hu SL, Zhao FR, Hu Q, et al. Meta-Analysis Assessment of GP210 and SP100 for the Diagnosis of Primary Biliary Cirrhosis. PLoS One 2014;9:e101916. [Crossref] [PubMed]
- Martins MJB, Batista AMA, Brito YNF, et al. Effect of Remote Ischemic Preconditioning on Systemic Toxicity and Ototoxicity Induced by Cisplatin in Rats: Role of TNF-α and Nitric Oxide. ORL J Otorhinolaryngol Relat Spec 2017;79:336-46. [Crossref] [PubMed]
- Wilasco MI, Uribe-Cruz C, Santetti D, et al. IL-6, TNF-α, IL-10, and nutritional status in pediatric patients with biliary atresia. J Pediatr (Rio J) 2017;93:517-24. [Crossref] [PubMed]
- De Simone V, Franzè E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015;34:3493-503. [Crossref] [PubMed]
- Xiong H, Wei L, Peng B. IL-17 stimulates the production of the inflammatory chemokines IL-6 and IL-8 in human dental pulp fibroblasts. Int Endod J 2015;48:505-11. [Crossref] [PubMed]
- McGovern JL, Nguyen DX, Notley CA, et al. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti–tumor necrosis factor antibody therapy. Arthritis Rheum 2012;64:3129-38. [Crossref] [PubMed]
- Taylor PR, Roy S, Leal SM, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat Immunol 2014;15:143-51. [Crossref] [PubMed]
- Kimura A, Kishimoto T. IL-6: Regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830-5. [Crossref] [PubMed]


