
Immunoregulatory role of IL-2/STAT5/CD4+CD25+Foxp3 Treg pathway in the pathogenesis of chronic osteomyelitis
Introduction
Chronic osteomyelitis (COM) is a chronic bone infection characterized by progressive osteonecrosis and dead bone formation, which is closely related to the persistent infectious inflammation after infection (1). A variety of studies have shown that COM patients have disordered T lymphocyte proliferation, and clinical and animal studies also reveal that IL-2 expression and CD4+CD25+Foxp3 Treg cells increase in the peripheral blood in case of COM. IL-2 can bind to IL-2R receptor on the CD25+CD4+Treg cells, thereby activating activated protein 5 (STAT5) which then bind to the gene promoter of Foxp3, a factor regulating Treg cell activity. This may elevate the immunosuppressive activity of Treg cells, and compromise the immune response of T lymphocytes to pathogen. Thus, IL-2 mediated regulation of CD4+CD25+Foxp3 Treg cells via STAT5 pathway may play a key role in the development of COM (2-5). To date, little is known about the role of IL-2/STAT5/CD4+CD25+Foxp3 Treg pathway in the pathogenesis of COM. This study aimed to investigate the immunoregulatory role of this pathway in the pathogenesis of COM.
Methods
Animals
A total of 48 Sprague-Dawley (SD) rats weighing 180–220 g were purchased from Shanghai Xipur-Bikai Experimental Animal Co., Ltd and given ad libitum access to water and food. Animals were housed for 1 week before the experiment. This study has been approved by the Ethics Committee of Tongde Hospital of Zhejiang Province.
Drugs and reagent
Rat IL-2 detection kit (BD Company, USA), hematoxylin (BASO, China; No: 714094), eosin (BASO, China; No: BA4099), Trizol (Invitrogen, USA; No: 1596-026), polymerase chain reaction (PCR) kit (Thermo, USA; No: #K0223), reversion transcription kit (Fermentas, USA; No: #K1622), STAT5 (Abcam, UK; No: ab16276), p-STAT5 antibody (Abcam, UK; No: ab32364), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (CST, USA; No: #5174), horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody (Beyoutime, China; No: A0208), HRP conjugated donkey anti-goat secondary antibody (Beyoutime, China; No: A0181), HRP conjugated goat anti-mouse secondary antibody (Beyoutime, China; No: A0216), and anti-human Foxp3 staining Kit (BD Company, USA; No: 560133) were used in the present study.
Instrument
Light microscope (ECLIPSE Ni, Nikon, Japan), pipette (Gilson company, USA), constant temperature oven (Shanghai Hengyi Scientific Instrument Co., Ltd., China), paraffin slicer (Leica company, Germany); microscopic image analysis system (DS-Ri2; Nikon, Japan), thermal cycler (ABI, USA); cryogenic centrifuge (Shanghai Luxiangyi Centrifuge Instrument Co., Ltd., China), vortex oscillator (Qingpu West Instrument Factory, China), electric homogenizer (FLUKO, China); electrophoresis instrument (BIO-RAD company, USA), electric transfer instrument (BIORAD company, USA); microplate reader (Labsystems, Finland), water bath (Leica company, Germany) and flow cytometer (Accuri C6; BD Company, USA) were used in the present study.
Establishment of animal model and grouping
The tibia was drilled and then inoculated with Staphylococcus (0.1 mL; 2×108/mL ATCC25934) aureus to induce COM (6). Four weeks later, computed tomography (CT) scanning was performed to assess the bone defects, bacteriological detection was done to assess focal infection, and the lesioned bone was collected for routine pathological examination, aiming to confirm the successful establishment of COM model. In sham-operated rats, the tibia was drilled but not inoculated with bacteria. Sampling was done at 1, 2 and 4 weeks (n=8 at each time point). All the animals were intragastrically administered with normal saline.
Hematoxylin and eosin (HE) staining
The tibia was collected at different time points, fixed in 4% paraformaldehyde, dehydrated in a series of ethanol, embedded in paraffin, sectioned, and deparaffinized, followed by hematoxylin and eosin staining. After mounting with neutral resin, sections were observed under a light microscope and representative images were captured for further analysis. Light microscope and microscopic image analysis system; 200× were used.
Detection of IL-2 expression by enzyme-linked immunosorbent assay (ELISA)
One, two and four weeks later, 5 mL of blood was collected from the abdominal aorta of each rat. Serum was taken after centrifugation and then processed according to the manufacturer’s instructions. The absorbance was detected with a microplate reader at 450 nm and IL-2 concentration was calculated based on the standard curve.
Detection of CD4+CD25+Foxp3 Treg cells in peripheral blood by flow cytometry
Single mononuclear cell suspension was prepared, followed by cell counting. The cell density was adjusted and then incubated with CD4, CD25 and Foxp3 antibodies according to manufacturer’s instructions. CD4+CD25+Foxp3+ cells were detected by flow cytometry.
Detection of Foxp3 and CTLA-4 expression by real-time PCR
Mononuclear cells were isolated from peripheral blood, and the magnetic beads were used to further sort CD25+CD4+Treg cells. Total RNA was extracted from CD25+CD4+Treg cells and RNA concentration was determined. Real-time PCR was performed according to the manufacturer’s instructions, and GAPDH served as an internal reference (Table 1).
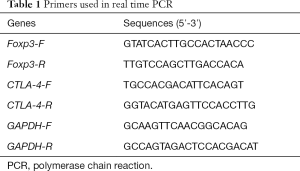
Full table
Detection of STAT5 and p-STAT5 expression by Western blotting
Total protein was extracted and the protein concentration was determined by bicinchoninic acid assay (BCA) assay. The protein expression of STAT5 and p-STAT5 was detected with routine protocol and GAPDH served as an internal reference. The optical density of each protein band was analyzed with Image J software and then normalized to that of GAPDH.
Statistical analysis
Data are expressed as mean ± standard deviation (
Results
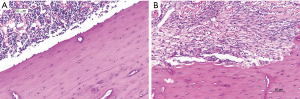
Morphology of tibia
Four weeks after the surgery, animals were euthanized for histological analysis. In COM group, the periosteum was thickened, and a large number of inflammatory cells were observed in the medullary cavity, and the bone tissues were destroyed although the continuity remained. In control group, the bone tissues were arranged closely, there was no obvious bone destruction, and infiltration of neutrophil was not observed in the medullary cavity (Figure 1).
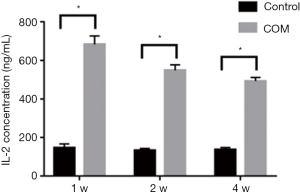
IL-2 contents
One, two and four weeks later, the IL-2 expression increased significantly in COM rats as compared to controls (P<0.001), suggesting the high IL-2 expression in the blood (Figure 2).
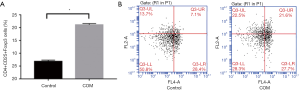
Proportion of CD4+CD25+Foxp3 Treg cells in peripheral blood
The proportion of CD4+CD25+Foxp3 Treg cells in the peripheral blood of COM group was significantly higher than in control group (P<0.001) (Figure 3).
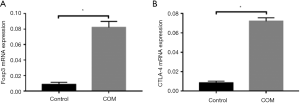
mRNA expression of Foxp3 and CTLA-4 in CD4+CD25+ Treg cells
In addition, Foxp3 and CTLA-4 expression were further detected in CD4+CD25+ Treg cells. Results showed the mRNA expression of Foxp3 and CTLA-4 in CD4+CD25+ Treg cells of COM group was significantly higher than in control group (P<0.001) (Figure 4).
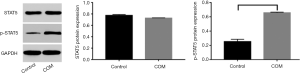
Protein expression of STAT5 and p-STAT5 in CD4+CD25+Foxp3 Treg cells
As shown in Western blotting, the p-STAT5 protein expression in COM group was significantly higher than in control group (P<0.001) (Figure 5).
Discussion
COM has complicated disease conditions, long natural course, high rate of surgical failure and high recurrence, and is also easy to cause chronic sinus, fracture, bone defect, or even severe amputation and life-threatening conditions if serious enough. In addition, COM requires multiple debridements and long-term use of antibiotics, which are costly and impose a heavy financial burden on patients and society. Thus, COM has been a challenge faced by orthopedic surgeons in clinical practice (7).
The persistent infectious inflammation in case of COM is closely related to bone destruction, in which immunoregulation plays an important role (8). Available studies have confirmed that T lymphocytes and their products are crucial for the formation and function of osteoclasts and osteoblasts (9). The abnormal proliferation of T lymphocytes is closely related to the persistent bone destruction in case of COM. In recent years, increasing attention has been paid to the role of CD25+CD4+ regulatory T lymphocytes (CD25+CD4+Treg cells) in the chronic bone infection (10). CD4+CD25+Foxp3 Treg cells are derived from CD4+ T cells, and the differentiation of initial CD4+ T cells into Treg cells is partially dependently on IL-2, which is essential for the survival, proliferation, and stability of Treg cells. IL-2 can activate the transcription factor STAT5, and the activated STAT5 binds to the non-coding sequence 2 (CNS2) of the conserved region of Foxp3 gene enhancer. Foxp3 is currently recognized as the most characteristic molecular marker of Treg cells and is also specific to Treg cells. The activation of Foxp3 is dependent on STAT5 signaling pathway (11-13). IL-2 is an important factor responsible for the conversion of CD4+ cells to CD4+CD25+Foxp3 Treg cells, and the elevation of IL-2 expression suggests the increase in CD4+CD25+Foxp3 Treg cells. Therefore, the IL-2/STAT5 signaling pathway can be considered as a key regulatory point in the differentiation of initial CD4+ T cells into Treg cells (14). STAT5 is a member of STATs family. As a transcriptional regulator widely expressed in human and murine tissues, STAT family proteins are mainly inactive in the cytoplasm of cells in absence of stimulation. The STAT is mainly composed of N-terminal domain, Src Homology 2 (SH2) domain and C-terminal transcriptional activation domain. When cytokines such as IL-2 bind to their specific receptors, it may trigger receptor dimerization, resulting in the recruitment of Janus kinase (JAK). The aggregated JAK kinase is self-activated by phosphorylation, then translocated into the nucleus and binds to Foxp3, inducing the conversion of CD4+ T cells into CD4+CD25+Foxp3 Treg cells. Treg cells may exert immunosuppressive effects via directly cell-cell contact, inhibiting metabolism in target cells, secreting inhibitory cytokines (such as IL-10 and TGF-β1) in the presence of cell surface molecules (such as CTLA-4). Treg cells play important roles in the maintenance of immune tolerance, autoimmune diseases, acute and chronic inflammation, and cancers (15,16). Therefore, the IL-2/STAT5/CD4+CD25+Foxp3 Treg pathway is also crucial for the pathogenesis of COM. The activation of IL-2/STAT5/CD4+CD25+Foxp3 Treg cells may enhance the immunosuppression of Treg cells, thereby compromising the immune response of T lymphocytes to pathogens, which results in the persistent infectious inflammation.
In order to further verify the immunoregulatory effects of this pathway in COM, COM model was established in rats. HE staining showed periosteal thickening, infiltration of a large number of inflammatory cells in the medullary cavity as well as bone disruption. This suggests successful establishment of COM animal model as demonstrated by the typical chronic inflammation. However, in control group, the bone tissues were closely arrangement, and there was no obvious bone destruction and infiltration of inflammatory cells in the medullary cavity (17). A variety of studies have shown the expression of IL-2 in the serum increases in patients with COM and COM animals (2-5). Therefore, at different time points, the blood was collected for the detection of serum IL-2 concentration. Results showed serum IL-2 concentration in COM group was significantly higher than in control group (P<0.001), which was consistent with previously reported (18). Flow cytometry is a tool used for rapid and accurate quantitative analysis of cells with monoclonal antibodies at the cellular level. In the present study, mononuclear cells were collected from the blood of COM rats and the proportion of CD4+CD25+Foxp3 Treg cells was determined by flow cytometry (19). Results showed significant different in the proportion of CD4+CD25+Foxp3 Treg cells between COM group and control group (P<0.001). This indicates the CD4+CD25+Foxp3 Treg cells increase, indicating the elevated immunosuppression. In addition, the mRNA expression of Foxp3 and CTLA-4 was also detected in CD4+CD25+Foxp3 Treg cells. Foxp3 is a characteristic molecule of Treg cells (20). CTLA-4 is a transmembrane receptor and a phenotype molecule of Treg cells, and CTLA-4 expression may reflect the Treg cell activation (21). In COM group, the mRNA expression of Foxp3 and CTLA-4 was also markedly higher than in control group (P<0.001). The mechanism underlying the STAT5 phosphorylation has been addressed above, p-STAT5 is the active form of STAT5. Although there was no significant difference in STAT5 expression between COM group and control group, the p-STAT5 expression in COM group was significantly higher than in control group (P<0.001). This suggests STAT5 pathway is activated, and the activated STAT5 may bind to Foxp3, exerting biological effects.
Taken together, IL-2/STAT5/CD4+CD25+Foxp3 Treg pathway plays a negative regulatory role in occurrence and development of COM. Moreover, pharmacological intervention may employed to interfere with the immunoregulation.
Acknowledgments
Funding: The study was supported by National Natural Science Foundation of China (81603644).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study has been approved by the Ethics Committee of Tongde Hospital of Zhejiang Province.
References
- Lan Y, Xie H, Shi Y, et al. NEMO binding domain peptide ameliorates inflammatory bone destruction in a staphylococcus aureus induced chronic osteomyelitis model. Mol Med Rep 2019;19:3291-7. [PubMed]
- Li P, Leonard WJ. Chromatin accessibility and interactions in the transcriptional regulation of T cells. Front Immunol 2018;9:2738. [Crossref] [PubMed]
- Sheikh V, Zamani A, Mahabadi-Ashtiyani E, et al. Decreased regulatory function of CD4+CD25+CD45RA+ T cells and impaired IL-2 signalling pathway in patients with type 2 diabetes mellitus. Scand J Immunol 2018;88:e12711. [Crossref] [PubMed]
- Wu Y, Tang Y, Liang X, et al. The role of increased frequency of treg cells in patients with chronic osteomyelitis. Orthopedics 2011;34:98. [PubMed]
- Yoon KS, Fitzgerald RH Jr, Sud S, et al. Experimental acute hematogenous osteomyelitis in mice. II. Influence of staphylococcus aureus infection on T-cell immunity. J Orthop Res 1999;17:382-91. [Crossref] [PubMed]
- Yan L, Jiang DM, Cao ZD, et al. Treatment of Staphylococcus aureus-induced chronic osteomyelitis with bone-like hydroxyapatite/poly amino acid loaded with rifapentine microspheres. Drug Des Devel Ther 2015;9:3665-76. [Crossref] [PubMed]
- Maffulli N, Papalia R, Zampogna B, et al. The management of osteomyelitis in the adult. Surgeon 2016;14:345-60. [Crossref] [PubMed]
- Wang Y, Wang J, Meng J, et al. Epigenetic modification mediates the increase of LAG-3+ T cells in chronic osteomyelitis. Inflammation 2017;40:414-21. [Crossref] [PubMed]
- Li Z, Xu Z, Duan C, et al. Role of TCF/LEF transcription factors in bone development and osteogenesis. Int J Med Sci 2018;15:1415-22. [Crossref] [PubMed]
- Chen Q, Zhang X, Xiong Y, et al. The CD25+/CD4+ T cell ratio and levels of CII, CIX and CXI antibodies in serum may serve as biomarkers of pristane-induced arthritis in rats and rheumatoid arthritis in humans. Comp Biochem Physiol C Toxicol Pharmacol 2019;217:25-31. [Crossref] [PubMed]
- Apert C, Romagnoli P, van Meerwijk JPM. IL-2 and IL-15 dependent thymic development of Foxp3-expressing regulatory T lymphocytes. Protein Cell 2018;9:322-32. [PubMed]
- Fan MY, Low JS, Tanimine N, et al. Differential roles of IL-2 signaling in developing versus mature tregs. Cell Rep 2018;25:1204-13.e4. [Crossref] [PubMed]
- Whitehouse G, Gray E, Mastoridis S, et al. IL-2 therapy restores regulatory T-cell dysfunction induced by calcineurin inhibitors. Proc Natl Acad Sci U S A 2017;114:7083-8. [Crossref] [PubMed]
- Shi H, Liu C, Tan H, et al. Hippo kinases Mst1 and Mst2 sense and amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity 2018;49:899-914.e6. [Crossref] [PubMed]
- Johnson JL, Jones MB, Cobb BA. Polysaccharide-experienced effector T cells induce IL-10 in FoxP3+ regulatory T cells to prevent pulmonary inflammation. Glycobiology 2018;28:50-8. [Crossref] [PubMed]
- Loh CY, Arya A, Naema AF, et al. Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication. Front Oncol 2019;9:48. [Crossref] [PubMed]
- Goto B, Iriuchishima T, Horaguchi T, et al. Therapeutic effect of photodynamic therapy using Na-pheophorbide a on osteomyelitis models in rats. Photomed Laser Surg 2011;29:183-9. [Crossref] [PubMed]
- García-Alvarez F, Navarro-Zorraquino M, Castro A, et al. Effect of age on cytokine response in an experimental model of osteomyelitis. Biogerontology 2009;10:649-58. [Crossref] [PubMed]
- Wu JH, Zhou M, Jin Y, et al. Generation and immune regulation of CD4+CD25-Foxp3+ T cells in chronic obstructive pulmonary disease. Front Immunol 2019;10:220. [Crossref] [PubMed]
- Zhu XW, Zhu HZ, Zhu YQ, et al. Foxp3 expression in CD4+CD25+Foxp3+ regulatory T cells promotes development of colorectal cancer by inhibiting tumor immunity. J Huazhong Univ Sci Technolog Med Sci 2016;36:677-82. [Crossref] [PubMed]
- Chen X, Du Y, Hu Q, Huang Z. Tumor-derived CD4+CD25+regulatory T cells inhibit dendritic cells function by CTLA-4. Pathol Res Pract 2017;213:245-9. [Crossref] [PubMed]


