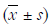Lower limb arterial intervention or autologous platelet-rich gel treatment of diabetic lower extremity arterial disease patients with foot ulcers
Introduction
Diabetic patients have been estimated to have a 15% risk of developing at least once foot ulcer during their lifetime, and approximately 40–80% of nontraumatic amputations are associated with diabetic foot ulcers (DFUs). Since DFUs are a serious complication of diabetes because of peripheral arterial disease, they pose a great challenge in the treatment of diabetes (1). According to Lancet, the number of patients with peripheral arterial disease in the world exceeded 200 million in 2010. Compared with nondiabetic patients, diabetic patients often have multivascular lesions. Lower extremity arterial disease (LEAD) and cardiovascular events often coexist, and LEAD is one of the most common risk factors for cardiovascular diseases: the lower the ankle brachial index (ABI) is, the worse the cardiovascular prognosis; and patients with lower limb multivascular lesions often experience a worse prognosis than those with only a single vascular lesion. Unfortunately, to a large extent, LEAD is an invisible disease, and most patients with LEAD seem to have a negative attitude and do not seek treatment because they are unaware of symptoms. Additionally, many studies have indicated that diabetic LEAD is a leading cause of DFUs and amputation, especially in China. China-DiaLEAD has declared that the prevalence of diabetic LEAD is estimated to be approximately 21.2%, and the severity increases with age and the duration of diabetes, leading to a higher risk of diabetic LEAD patients with foot ulcers and amputation than in nondiabetic individuals (2). Therefore, more attention should be paid to diabetic LEAD patients with foot ulcers.
At present, the possible treatment options of diabetic LEAD patients with foot ulcers include drugs, debridement, dressing, etc. For the complex lesions of diabetic LEAD patients with foot ulcers, the treatment effect is unsatisfactory; it often leads to poor outcomes, such as amputation, morbidity and mortality. In recent years, lower extremity arterial intervention (angioplasty), percutaneous transluminal angioplasty (PTA) and stent implantation have been shown to restore the lower limb blood supply. However, in diabetic LEAD patients, it is difficult to restore the blood supply to diffuse artery disease, especially below the knee. Although many novel approaches, such as drug-coated balloons and cutting balloon angioplasty, have been used for therapy, they still hold high rates of restenosis, recurrence and amputation (3).
As one of the treatment options for diabetic wounds, autologous platelet-rich gel (APG) therapy can effectively improve the healing of DFUs. The mechanism of APG in wound reparation is mainly associated with concentrated platelets and high concentrations of growth factors. APG upregulates the levels of various growth factors; improves the surrounding microenvironment; regulates the balance between tissue inhibitors of metalloproteinases (TIMPs) and matrix metalloproteinases (MMPs); reduces the degradation of local collagen, the extracellular matrix and growth factors; provides a fibrin scaffold; prolongs the action time of growth factors and bonding wound margins; reduces the release of surface tension; releases antimicrobial active substances; inhibits the infection of local microorganisms; promotes the healing of ulcers; and has little adverse effects (4). However, there are few reports on diabetic LEAD patients with foot ulcers. Therefore, one question to be addressed is whether APG can improve wound healing and reduce amputation. Another question to be addressed, on the basis of traditional treatment, is whether lower limb vascular intervention or APG contributes more to wound healing and the amputation rate in diabetic LEAD patients with foot ulcers. Therefore, we compared the treatment effects and amputation rate between lower extremity vascular intervention and APG in diabetic LEAD patients with foot ulcers.
Methods
Subjects and group classification
The study protocol was approved by the Southwest Hospital of the Army Medical University Institutional Review Board and conformed to the standards of the Declaration of Helsinki and is registered at ClinicalTrials.gov (NCT 03248466). Inclusion criteria were as follows: (I) DFU and hemoglobin A1c (HbA1c) >6.5%; (II) between the ages of 18–85 years; (III) Wagner classification of diabetic foot grade 2–4; (IV) ABI <0.9 and color Doppler ultrasonography suggested LEAD. Exclusion criteria were as follows: (I) nondiabetic patients or patients with type 1 diabetes [serum islet cell antibodies (ICA)- or glutamic acid decarboxylase antibody (GADA)-positive autoimmune diabetes] or a special type of diabetes (e.g., gestational diabetes); (II) patients who had severe liver or renal failure; (III) severe infections in patients and patients who had cerebrovascular disease or heart failure; (IV) age >85 years or <18 years. All patients were recruited from the First Affiliated Hospital of the Army Medical University after receiving approval for each treatment. Amputation was determined by the orthopedists for possible treatment as needed in the immediate treatment period and based on the patient’s willingness. These criteria were determined by each patient’s physicians prior to the patient being enrolled in the study.
There were 99 diabetic LEAD patients with foot ulcers between June 2012 and January 2018 who were recruited. These patients have been included in the standard diagnostic criteria of the World Health Organization (WHO) 1999 (judgment was made according to the case history, physical examination, medication, hormone history, blood testing, etc.). All of the patients were diagnosed by physicians and were advised to receive lower extremity vascular interventional or APG treatment in addition to traditional treatment. We used random number assignment. A total of 82 patients were enrolled in the study. Among them, 30 patients were randomly assigned and agreed to receive lower extremity vascular intervention on the basis of traditional treatment (group B), 31 patients were randomly assigned and agreed to receive traditional treatment (group A), and 21 patients were randomly assigned and agreed to receive APG treatment on the basis of traditional treatment (group C). A total of 17 patients withdrew from the study because they disagreed with the assigned treatment. Lower limb arterial intervention and APG treatment have been previously described. According to the size of the wound, we collected 8–50 mL of venous blood, which was placed in a vacuum blood vessel containing 3.0% sodium citrate, and the first centrifugation was as follows: centrifugal force 160 ×g, centrifugal time 20 minutes. After centrifugation, all supernatant fluid, the albuginea layer and the erythrocyte supernatant were absorbed and transferred to a centrifugal tube for the second centrifugation, as follows: centrifugal force 400 ×g, centrifugal time 15 minutes, centrifugal radius 13 cm, discarded supernatant fluid 3/4 (the remaining 1/4 was platelet-rich plasma). Thrombin was injected into calcium chloride, and the platelet-rich plasma and thrombin calcium were mixed at a 10:1 ratio to yield APG. After the ulcer was thoroughly debrided, APG was evenly sprayed onto the ulcer surface, and after stabilization, a Vaseline gauze was placed on the surface. We changed the dressing once a week (5,6).
Study methods
The age, height, weight, body mass index (BMI), blood pressure (BP), and course of disease were measured using the International Collaborative Study on Hypertension in Blacks (ICSHIB) standardized protocol. Fasting plasma glucose (FPG), HbA1c, fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein (HDL) were tested by certified laboratories, and the coefficients of variation within and between batches for all parameters were <5%. The English Huntleigh MD2 diabetes screening and diagnosis box was applied to the ABI evaluation. Transcutaneous oxygen partial pressure (TcPO2) was tested with a multichannel TcPO2 analyzer TCM400 from a radiometer company as a reference (7). The basic treatment program included glycemic control (insulin or oral hypoglycemic agents), antibiotic therapy, surgical debridement and drainage (removing corrupt tissues and drainage but not having minor or major amputation), microcirculation improvement (alprostadil), neurotropic therapy (mecobalamin and α-lipoic acid), and support treatment; all patients received basic treatment (6). Whether or not to perform an amputation and the level of amputation were determined according to the judgment of the orthopedist and the patient’s willingness. We evaluated each diabetic foot with the wound, ischaemia, and foot infection (WIFI) classification (5). We also used Armstrong and Frankberg classifications to classify surgical types into 1–4 levels (8). All these data were recorded as baseline.
Follow-up visits took place during outpatient visits 6 months after leaving the hospital. During follow-up, we collected data on the rate of DFU healing, the rate of amputation, the rate of recurrence, the ABI, TcPO2, hospital admissions, and other adverse events.
Safety variables included adverse events such as postoperative infection, transient fever, allergic reaction, postoperative pain, postoperative hemorrhage and patient-reported hypoglycemic episodes. A serious adverse event was defined as an adverse event that resulted in major morbidity, all-cause mortality, hospitalization, amputation, disability, or an event that required medical or surgical intervention to prevent one of the other outcomes. A severe adverse event was defined as an adverse event causing unacceptable and considerable interference with the patient’s daily activities.
Data collection and outcome measures
Primary endpoints included the rate of amputation, including major amputation (an amputation above the ankle) and minor amputation (an amputation under the ankle), and the rate of wound healing after 6 months of follow-up. Complete wound healing was defined as complete re-epithelization. Wounds leading to major amputation were classified as “not healed.” Wounds that healed after minor amputation were classified as “healed.” Recurring ulcers after initial healing were classified as healed but are reported under the secondary outcome “ulcer recurrence”. Readmission to the hospital for DFUs after initial healing was classified as “rehospitalization” (9).
Secondary endpoints included the ABI and TcPO2 after 6 months of follow-up; additional revascularization on the index limb that was not planned at the beginning of the study; new or recurrent ulcers; and (serious) adverse events, which were defined as any untoward medical occurrence, including major morbidity, all-cause mortality, ulcer recurrence, and rehospitalization.
Statistical analyses
The statistical software SPSS 19.0 was employed for statistical analyses. P<0.05 indicates statistical significance. The data are shown as
Results
Comparison of clinical data among the three groups
No significant difference was found in age, FPG, HbA1c, TC, TG, LDL-C, HDL, or BP between the groups. There were no significant differences in the ABI or TcPO2 between the three groups (see Table 1).
Comparison of each parameter before and after treatment (see Table 2)
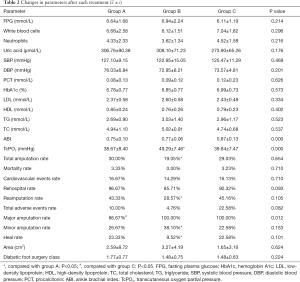
Our research suggested that after 6 months of each intervention, some indicators, such as leukocyte count, ulcer area, ABI, TcPO2, and blood lipid profiles, were significantly improved. There was no difference in the ABI between groups A and B (P>0.05), and the ABI in group C was improved (P<0.05). There was no significant difference in TcPO2 between groups A and C (P>0.05), and TcPO2 in group B was improved (P<0.05). There was no significant difference in the leukocyte count, amputation rate, mortality, incidence of adverse cardiovascular events, readmission rate or reamputation rate (P>0.05), and there was no significant difference in the total clinical outcome (P>0.05). There was no significant difference in mortality or the incidence of adverse cardiovascular events (P>0.05). There was no difference in the healing rate between groups A and C (P>0.05), but the healing rate of group B was increased significantly (P<0.05).
In addition, we further analyzed the station of amputation and the healing of wounds. There was no difference in the major amputation rate between groups A and B (P>0.05), and that in group C was decreased significantly (P<0.05). There was no difference in the major amputation rate between groups A and C (P>0.05). The minor amputation rate in group B was increased significantly (P<0.05). This result indicates that lower limb vascular intervention and APG treatment can significantly improve DFUs.
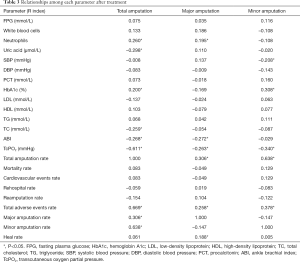
Relationships among changes in each parameter at 6 months after treatment (see Table 3)
Full table
Our research also provided the correlation between each indicator with major amputation, minor amputation, and total amputation after 6 months of each intervention. Neutrophils (R=0.195, P<0.05), the ABI (R=–0.272, P<0.05), TcPO2 (R=–0.263, P<0.05), total amputation (R=0.306, P<0.05), total adverse events (R=0.258, P<0.05), and healing (R=0.188, P<0.05) were associated with major amputation; systolic blood pressure (SBP) (R=–0.208, P<0.05), HbA1c (R=0.308, P<0.05), TcPO2 (R=–0.340, P<0.05), total amputation (R=0.638, P<0.05), and total adverse events (R=0.378, P<0.05) were associated with minor amputation; neutrophils (R=0.260, P<0.05), uric acid (R=–0.298, P<0.05), HbA1c (R=–0.200, P<0.05), TC (R=–0.259, P<0.05), the ABI (R=–0.268, P<0.05), TcPO2 (R=–0.611, P<0.05), total adverse events (R=0.669, P<0.05), major amputation (R=0.306, P<0.05), and minor amputation (R=0.638, P<0.05) were associated with total amputation.
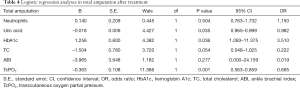
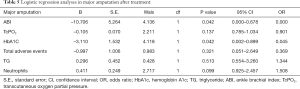
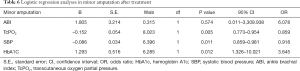
Logistic regression analyses in each parameter after treatment (see Tables 4-6)
Full table
Full table
Full table
Uric acid, glycosylated hemoglobin, TC, and TcPO2 were identified as the risk factors for total amputation, of which TcPO2 was the most significant. The ABI and glycosylated hemoglobin were identified as major risk factors for amputation, of which the ABI was the most significant. TcPO2, SBP and glycosylated hemoglobin were identified as the risk factors for minor amputation, of which TcPO2 was the most significant.
Discussion
In China, LEAD is the leading pathophysiological basis of DFUs, and it is also the most important factor in preventing the healing and amputation of DFUs (10,11). The ABI is a useful diagnostic measurement of LEAD. However, the ABI in diabetic LEAD patients with foot ulcers is often highly evaluated because it is often associated with vascular calcification and impaired elasticity (7); therefore, a more reliable diagnostic measurement is needed. TcPO2 reflects the status of oxygen metabolism and the microcirculation in diabetic LEAD patients with foot ulcers. It is the most common measurement used to evaluate the blood supply level of LEAD and to determine whether patients need to undergo artery reconstruction, ulcer healing and amputation (5). TcPO2 <30 mmHg can be used as a critical value for the diagnosis of diabetic LEAD patients with foot ulcers and for predicting the amputation of ulcerations.
Because of the complicated pathogenesis of DFUs, traditional treatment is often useless, and the ulcer is difficult to heal. Researchers have aimed to improve the blood supply to the lower extremities by angioplasty techniques, such as balloon dilatation, stents, drug-coated balloons, a plaque spin cutting system and a thrombus aspiration system. However, because of the poor coagulation and high oxidative stress environment in diabetes, the angioplasty in LEAD is difficult to manipulate, which often leads to a poor vascular situation and a difficult operation (3). On the other hand, since diabetic LEAD is usually performed below the ankle, angioplasty often improves the macrocirculation and has less effect on the microcirculation; therefore, the restenosis rate, the incidence of rehospitalization and the reamputation of diabetic LEAD patients with foot ulcers are still high (12,13).
Research has also indicated that the use of APG may affect the treatment of DFUs (4,14). APG provides a variety of growth factors and cytokines that improve the blood supply of the microcirculation, improves local inflammation and immune function, inhibits bacterial growth, promotes wound healing and improves clinical outcomes (15,16). These findings give rise to several questions, such as which is more important in the evaluation of diabetic LEAD patients with foot ulcers: the macrocirculation or the microcirculation, ABI or TcPO2; does the macrocirculation or microcirculation play a more important role in wound healing and which reduces the amputation rate; and does APG more effectively improve wound healing and reduce the amputation rate in diabetic LEAD patients with foot ulcers than angioplasty?
Our research suggested that the leukocyte count, ulcer area, ABI, TcPO2, and blood lipid profiles were significantly improved in each group after treatment. There was no significant difference in the leukocyte count, amputation rate, mortality, incidence of adverse cardiovascular events, readmission rate, reamputation rate, mortality, total clinical outcomes or incidence of cardiovascular events (P>0.05), which suggested no differences between the three groups. There was no difference in the ABI between groups A and B (P>0.05), and the ABI in group C was improved (P<0.05). There was no significant difference in TcPO2 between groups A and C (P>0.05), but TcPO2 in group B was improved (P<0.05). This finding suggested that basal and APG treatment had a greater effect on the improvement in TcPO2, and that basal and angioplasty treatment may have an effect on the improvement in the ABI.
In addition, we further analyzed the amputation rates and healing of wounds. There was no difference in the major amputation rate between groups A and B (P>0.05), but it was decreased significantly (P<0.05) in group C, while there was no difference in the minor amputation rate between groups A and C (P>0.05), but it was increased significantly (P<0.05) in group B. There was no difference in the heal rate between groups A and C (P>0.05), but it was increased significantly (P<0.05) in group B. The above data indicate that lower limb arterial intervention improves the ABI and reduces major amputation, but it does not improve minor amputation or wound healing; APG improves TcPO2, reduces minor amputation, and improves wound healing, but it does not improve the major amputation of DFUs.
We also analyzed the correlation between major amputation, minor amputation, and total amputation with each indicator. Neutrophils (R=0.195, P=0.042), the ABI (R=–0.272, P=0.008), TcPO2 (R=–0.263, P=0.010), total amputation (R=0.306, P=0.003), total adverse events (R=0.258, P=0.010), and healing (R=0.188, P=0.047) were associated with major amputation; SBP (R=–0.208, P=0.033), HbA1c (R=0.308, P=0.003), TcPO2 (R=–0.340, P=0.001), total amputation (R=0.638, P=0.000), and total adverse events (R=0.378, P=0.000) were associated with minor amputation; neutrophils (R=0.260, P=0.010), uric acid (R=–0.298, P=0.004), HbA1c (R=–0.200, P=0.038), TC (R=–0.259, P=0.010), the ABI (R=–0.268, P=0.008), TcPO2 (R=–0.611, P=0.000), total adverse events (R=0.669, P=0.000), major amputation (R=0.306, P=0.003), and minor amputation (R=0.638, P=0.000) were associated with total amputation.
We analyzed the risk factors for each major amputation, minor amputation, and total amputation. For major amputation, the ABI and HbA1c are risk factors, of which the ABI is the most significant (B=–10.706), because major amputation is significantly associated with macrocirculation, and the ABI, which reflects the macrocirculation, is significantly associated with major amputation (17). Therefore, we conclude that the ABI is more accurate in reflecting the needs of major amputation, and angioplasty improves the ABI and major amputation. Regarding minor amputation, TcPO2, SBP and HbA1c were identified as risk factors, with TcPO2 being the most significant (B=–0.152), because minor amputation is more associated with the local microcirculation; therefore, TcPO2, which reflects the local microcirculation, is significantly associated with minor amputation (18,19). The lower TcPO2 is, the more the patient is inclined to have minor amputation, and APG reduced minor amputation by improving TcPO2 (19,20). Therefore, we conclude that TcPO2 is more accurate in reflecting the needs of minor amputation and that APG improves TcPO2 and minor amputation. Regarding total amputation, uric acid, glycated hemoglobin, TC, and TcPO2 were identified as the risk factors, among which TcPO2 was the most significant (B=–0.363). Since minor amputation accounted more for total amputation than major amputation, TcPO2, which is more associated with minor amputation, was also significantly associated with total amputation (18-20). The above results suggest that major amputation is associated with the ABI, and angioplasty therapy has a greater effect on the improvement in the macrocirculation and ABI and reduced major amputation. Minor amputation was significantly associated with TcPO2, APG had a greater effect on the improvement in the microcirculation and TcPO2 and significantly reduced minor amputation.
One limitation of this study is that the sample size was limited. Another potential limitation is that the study was conducted at a single institute, which may result in bias.
This study demonstrates that in diabetic LEAD patients with foot ulcers, major amputation is mainly associated with the ABI, while minor amputation is significantly associated with local TcPO2. Angioplasty mainly improves the macrovasculature and the ABI and reduces the incidence of major amputation, whereas APG mainly improves the microcirculation and local TcPO2 and reduces the incidences of minor and total amputations. We will increase the sample size and the number of research centers to explore the mechanism of angioplasty and APG and to determine which better improves amputation and wound healing to clarify the wound healing of DFUs.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81370885) and The Clinical Trial Project of the First Affiliated Hospital of the Third Military Medical University in 2016 (SWH2016JSTSYB-11).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Southwest Hospital of the Army Medical University Institutional Review Board and conformed to the standards of the Declaration of Helsinki and is registered at ClinicalTrials.gov (NCT 03248466).
References
- Wu Q, Chen B, Liang Z. Mesenchymal stem cells as a prospective therapy for the diabetic foot. Stem Cells Int 2016;2016:4612167.
- Zhang X, Ran X, Xu Z, et al. Epidemiological characteristics of lower extremity arterial disease in Chinese diabetes patients at high risk: a prospective, multicenter, cross-sectional study. J Diabetes Complications 2018;32:150-6. [Crossref] [PubMed]
- Khor BYC, Price P. The comparative efficacy of angiosome-directed and indirect revascularisation strategies to aid healing of chronic foot wounds in patients with co-morbid diabetes mellitus and critical limb ischaemia: a literature review. J Foot Ankle Res 2017;10:26. [Crossref] [PubMed]
- Li L, Chen D, Wang C, et al. The effect of autologous platelet-rich gel on the dynamic changes of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in the diabetic chronic refractory cutaneous ulcers. J Diabetes Res 2015;2015:954701.
- Bakker K, Apelqvist J, Lipsky BA, et al. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 2016;32 Suppl 1:2-6. [Crossref] [PubMed]
- Wu Q, Lei X, Chen L, et al. Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: a case report. Ann Transl Med 2018;6:307. [Crossref] [PubMed]
- Deng W, Dong X, Zhang Y, et al. Transcutaneous oxygen pressure (TcPO2): a novel diagnostic tool for peripheral neuropathy in type 2 diabetes patients. Diabetes Res Clin Pract 2014;105:336-43. [Crossref] [PubMed]
- Armstrong DG, Frykberg RG. Classifying diabetic foot surgery: toward a rational definition. Diabet Med 2003;20:329-31. [Crossref] [PubMed]
- Santema KTB, Stoekenbroek RM, Koelemay MJW, et al. Hyperbaric oxygen therapy in the treatment of ischemic lower- extremity ulcers in patients with diabetes: results of the DAMO2CLES multicenter randomized clinical trial. Diabetes Care 2018;41:112-9. [PubMed]
- Sayampanathan AA, Cuttilan AN, Pearce CJ. Barriers and enablers to proper diabetic foot care amongst community dwellers in an Asian population: a qualitative study. Ann Transl Med 2017;5:254. [Crossref] [PubMed]
- Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †. Ann Med 2017;49:106-16. [Crossref] [PubMed]
- Palena LM, Diaz-Sandoval LJ, Gomez-Jaballera E, et al. Drug-coated balloon angioplasty for the management of recurring infrapopliteal disease in diabetic patients with critical limb ischemia. Cardiovasc Revasc Med 2018;19:83-7. [Crossref] [PubMed]
- Vouillarmet J, Bourron O, Gaudric J, et al. Lower-extremity arterial revascularization: Is there any evidence for diabetic foot ulcer-healing? Diabetes Metab 2016;42:4-15. [Crossref] [PubMed]
- Ahmed M, Reffat SA, Hassan A, et al. Platelet-rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg 2017;38:206-11. [Crossref] [PubMed]
- Chen L, Wang C, Liu H, et al. Antibacterial effect of autologous platelet-rich gel derived from subjects with diabetic dermal ulcers in vitro. J Diabetes Res 2013;2013:269527.
- Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of the diabetic foot. Diabetes Obes Metab 2015;17:435-44. [Crossref] [PubMed]
- Chang LH, Hwu CM, Chu CH, et al. The ankle brachial index exhibits better association of cardiovascular prognosis than non-high-density lipoprotein cholesterol in type 2 diabetes. Am J Med Sci 2016;351:492-8. [Crossref] [PubMed]
- Boyko EJ, Seelig AD, Ahroni JH. Limb- and person-level risk factors for lower-limb amputation in the prospective seattle diabetic foot study. Diabetes Care 2018;41:891-8. [Crossref] [PubMed]
- Wang Z, Hasan R, Firwana B, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg 2016;63:29S-36S.e1-2.
- Fossaceca R, Guzzardi G, Cerini P, et al. Endovascular treatment of diabetic foot in a selected population of patients with below-the-knee disease: is the angiosome model effective? Cardiovasc Intervent Radiol 2013;36:637-44. [Crossref] [PubMed]