
The effects of citrate dialysate in hemodialysis on polymorphonuclear elastase interaction with tissue factor and its inhibitor
Introduction
As the incidence of chronic kidney disease (CKD) steadily increase worldwide, hemodialysis (HD) is widely used for treating end-stage renal disease (ESRD). Adequate anticoagulation is a prerequisite for HD both to prevent clotting in the extracorporeal circuit (1), influencing the safety and efficacy of HD therapy. In China, there are three common anticoagulation means clinically, including unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and ‘no heparin,’ where LMWH is the prioritized option in maintenance HD. However, CKD patients already have an increased risk of excess bleeding, such as platelet dysfunction caused by uremia (2,3), let alone the potentially fatal heparin-induced thrombocytopenia type II and ‘no heparin’-induced consumption of platelet when receiving dialysis (4,5). Regional citrate anticoagulation has been tested on dialysis, but it might lead to potentially disastrous complications, such as citrate accumulation presenting metabolic acidosis, hypernatremia, and citrate net overload presenting metabolic alkalosis (6). The use of anticoagulation during HD remains a problem for ESRD patients with a high risk of bleeding complications. A new, safer, and more effective anticoagulation mean in HD is required.
Recently, many scholars have focused on tissue factor (TF), which initiates the extrinsic pathway of blood coagulation (4) and plays an important role in atherosclerosis (7,8). It was recently reported that adherent cells from the dialysis membrane were mainly constituted by activated polymorphonuclear neutrophils (PMNs), which triggered thrombin generation in a TF-dependent manner (9). The tissue factor pathway inhibitor (TFPI) is the only endogenous protein that effectively inhibits TF-factor VIIa in a factor Xa-dependent manner and dampens the activation of clotting through the extrinsic pathway under physiological conditions (10,11). Moreover, the serine proteases enhance TF- and factor XII-dependent coagulation in a process involving local proteolysis of the coagulation suppressor TFPI (12,13). Noteworthy, both UFH and LMWH induced a significantly enhanced secretion of TFPI (14) and the aggregation of human PMNs (15,16).
This study aims to investigate, excluding the effect of heparin on TFPI and PMNs, whether HD affects the level of TF, TFPI, and polymorphonuclear elastase (PMNE) in ESRD patients. Also, to analyze the reaction of TF, TFPI, and PMNE in ESRD patients throughout a single HD session and to explore whether TF or TFPI can be a new target for HD anticoagulation.
Methods
Study population
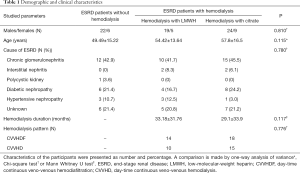
Thirty-three selected ESRD patients (CKD5d), who had already undergone hemodialysis for >3 months and used citrate for anticoagulation for >3 days, were enrolled in the study (citrate group). They were compared with age, sex and etiology-matched ESRD patients who had undergone hemodialysis for >3 months using LMWH as anticoagulation (LMWH group) (n=24) and those who had never received dialysis (control group) (n=28). Each case of ESRD was diagnosed with the calculated estimated glomerular filtration rate (eGFR) <10 mL/min/1.73 m2 using the simplified Modification of Diet in Renal Disease (MDRD) formula and was present for >3 months (17). Subjects were excluded from the study if they presented with any of the following diseases: malignancy, hyperlipidemia (cholesterol concentrations >6.21 mmol/L), advanced liver disease, contraceptive, or blood transfusion within three days before the study. The etiology of ESRD patients are displayed in Table 1.
Full table
The study was approved by the Research Ethics Committee of West China Hospital of Sichuan University (2016-188), and informed consent was obtained from all participants.
Dialysis details
Hemodialysis was applied using a temporary femoral venous catheter (n=36) or a permanent internal jugular venous cuff catheter (n=21). All these patients used polyacrylonitrile (AN69) as the dialysis membrane and were maintained on low-flux (130–200 mL/min) intermittent renal replacement therapy. For the citrate group, anticoagulation was carried out using sodium citrate (4.0%) continuously infused at a speed of 130–200 mL/h and extra sodium bicarbonate (5%) at 100–170 mL/h. For the LMWH group, a single i.v. bolus of LMWH at the start of hemodialysis (range, 1,000–2,500 IU) was followed by a constant infusion (range, 100–250 IU) and extra sodium bicarbonate (5%) at 190–260 mL/h. There were two dialysis patterns: day-time continuous veno-venous hemodialysis (CVVHD) and day-time continuous veno-venous hemodialysis filtration (CVVHDF) (Table 1). Correspondingly, the displacement velocity was 1,500/1,500 mL/h and 3,000 mL/h, and all adopted post-dilution.
Blood collection
Blood samples from hemodialysis patients were collected before a single dialysis session and at hours (h) 1, 2, and 5 during the hemodialysis. Residual containing fluid was removed from catheters before collection of the first sample. When collecting blood samples during the hemodialysis, we shut off the peripheral pump of the dialyzer for 3 min except for the blood pump and then collected blood samples directly from the arterial line on the machine. Fasting venous blood samples were taken from the antecubital vein in the morning from the control of ESRD patients. Citrated-plasma samples were prepared conventionally, centrifuged (15 minutes, 3,000 ×g), aliquoted and stored at −80 °C until the assay.
Analytical procedures
Plasma levels of TF and TFPI were measured in duplicate by enzyme-linked immunosorbent assays (ELISA) using commercially available standard kits supplied by R&D Systems, Inc., USA. The plasma level of PMNE was measured by ELISA kit purchased from Life Technologies, Carlsbad, USA.
Statistical analysis
Quantitative data followed the normal distribution and were expressed as the mean ± SD (standard deviation) in tables or mean ± SEM (standard error of the mean) in figures. Characteristics of the participants were first presented as numbers and percentages. The data obtained at different time points were corrected by hematocrit. Chi-square test, one-way analysis of variance (one-way ANOVA) or Mann Whitney U test were used to adjust models controlling for sex, etiology, and age. Comparisons of the TF, TFPI, and PMNE plasma levels between the three groups and the levels at different time points were performed using one-way analysis of variance or Kruskal-Wallis H test. Bivariate correlation analysis was applied to explore the association between measured plasma constituents before hemodialysis and clinical indexes and the correlation of TF, TFPI, and PMNE plasma levels at each time point during hemodialysis. All statistical analyses were performed using IBM SPSS software SPSS 24. P<0.05 was considered statistically significant.
Results
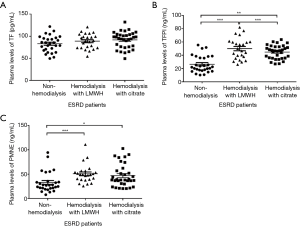
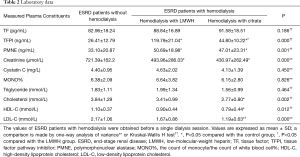
ESRD patients’ demographic characteristics did not differ significantly between the three groups (Table 1). Compared with the control subjects, patients on hemodialysis with LMWH and citrate showed significant higher plasma concentrations of TFPI (P=0.000, P=0.002, respectively) and PMNE (P=0.001, P=0.02, respectively) under baseline conditions (Figure 1). However, TF plasma levels (P=0.186) between the three groups were similar (Figure 1A). Also, we found under baseline conditions that the cholesterol of ESRD patients in the citrate group was lower than that of the control group, including total cholesterol (P=0.002), high-density lipoprotein cholesterol (HDL-C) (P=0.009), and low-density lipoprotein-cholesterol (LDL-C) (P=0.000). The clinical indexes of the studied groups are shown in Table 2.
Full table
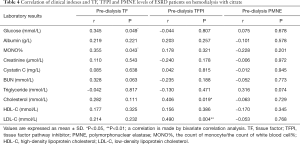
The results of factors correlating with plasma TF, TFPI, and PMNE levels in ESRD patients before hemodialysis are presented in Tables 3,4. We did not find any correlation between the pre-dialysis TF, TFPI, PMNE levels, and clinical indexes in the LMWH group (Table 3). However, in the citrate group, the pre-dialysis TF level was significantly correlated to glucose and monocyte % (r=0.345 and r=0.355, respectively; P<0.05). Also, the pre-dialysis TFPI level was positively associated with cholesterol (r=0.406, P=0.019) and LDL-C (r=0.490, P=0.004), whereas we did not find any clinical index that was correlated to the PMNE level before hemodialysis (Table 4).

Full table
Full table
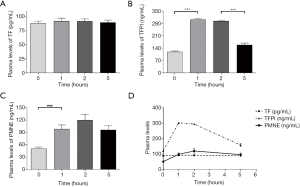
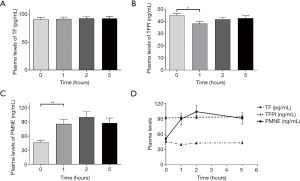
As the different time points were measured, presented in Table 5, showed that the plasma levels of TFPI and PMNE fluctuated obviously (P=0.000, P=0.000, respectively) during HD with LMWH, but TF did not change significantly (P=0.935). Further analysis showed that plasma TF levels did not vary obviously (Figure 2A); plasma TFPI levels ascended significantly at 1 h (P=0.000), descended slightly at 2 h (P=0.364) and significantly at 5 h (P=0.000) (Figure 2B); the plasma levels of PMNE increased significantly at 1 h (P=0.000) and continued to rise at 2 h but did not reach a significant level (P=1.000) (Figure 2C). The trends of TF, TFPI and PMNE in the LMWH group were shown in Figure 2D. For the citrate group, we found that the plasma levels of PMNE had changed significantly throughout hemodialysis (P=0.000), but the overall changes of TF (P=0.986) and TFPI (P=0.088) did not reach statistical significance (Table 6). Further analysis found that the change of plasma TF levels was not obvious, either (Figure 3A); plasma TFPI levels declined modestly at 1 h (P=0.012) (Figure 3B); the plasma levels of PMNE increased significantly at 1 h (P=0.008) and continued to rise at 2 h but did not reach statistical significance (P=1.000), then descended slightly at 5 h (P=1.000) (Figure 3C). The trends of TF, TFPI and PMNE in the citrate group were shown in Figure 3D.

Full table

Full table
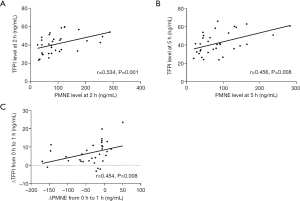
We used bivariate correlation analysis to explore the correlation of TF, TFPI, and PMNE plasma levels at each time point during hemodialysis. There was a possible correlation between TFPI levels and PMNE levels throughout the hemodialysis session with citrate, especially at 2 h (r=0.534, P=0.001) and 5 h (r=0.456, P=0.008), as shown in Figure 4. Furthermore, the change of the plasma PMNE level between 0 and 1 h was associated with the change of the plasma TFPI level between 0 and 1 h (r=0.454, P=0.008) (Figure 4C). However, there was no such change and correlation for TF and the LMWH group.
Also, we divided the experimental group into CVVHD group and CVVHDF group according to the different HD modes and analyzed whether there were differences in TF, TFPI and PMNE plasma levels at different time points in the dialysis process between the two groups. No significant difference was found between CVVHD and CVVHDF groups.
Discussion
The major finding of the present study is that hemodialysis affected the plasma levels of TFPI and PMNE of ESRD patients but had minimal effect on plasma TF. Moreover, we found that, excluding the effect of heparin on TFPI and PMNs, PMNE might play a role in anticoagulative activity through TFPI during hemodialysis.
Numerous studies indicate that, compared to healthy volunteers, plasma levels of TF and TFPI are higher in patients undergoing maintenance hemodialysis therapy (18-20). However, the variation of TF plasma levels during hemodialysis is inconsistent, as TF levels at times increase (21) or do not (18,19). It is contentious whether dialysis affects TF. Our study demonstrated that there was no difference in TF levels between ESRD patients with or without hemodialysis and that the TF plasma levels didn’t change during HD no matter what category of anticoagulation was adopted. These data suggest that a decreased kidney function is associated with increased TF levels, but not HD. TF is associated with enhanced oxidative stress (22,23), endothelial injury (24) and uremic solutes (25,26) in chronic kidney disease. A recent article reported that advanced oxidation protein products (AOPPs) and serum TF correlate with kidney function. ESRD patients who are on HD had significantly higher serum levels of AOPPs and TF compared to patients after kidney transplantation with a decent GFR (27). The study indicated that TF was still strongly associated with oxidative stress caused by renal insufficiency in HD patients. It was reported that the dialyzer membrane triggered TF expression of activated PMNs (9), but, in our study, the plasma levels of TF were not correlated with PMNE, which was a marker of activated PMNs. A possible reason for this phenomenon could be that TF was expressed richly enough because of renal insufficiency; consequently, the effect of dialysis on the tissue factor is weak. Thus, we speculate that the change in plasma TF level may be associated with chronic renal failure superior to HD. Also, the TF level of pre-dialysis was correlated with glucose and MONO%, which is similar to that of previous studies (10).
Several studies found that TFPI levels tended to rise significantly from minute 15 and minute 60 onward during hemodialysis (21,28,29), which is similar to the trend of the LMWH group in our study. There is no doubt that the use of heparin can significantly increase the TFPI level in hemodialysis patients. On the contrary, in the citrate group of our research, the plasma TFPI level decreased slightly at 1 h. It is noteworthy that all the previous studies used heparin for anticoagulation, while we used citrate. No literature has reported that citrate may affect TFPI, so it is considered that HD may affect the plasma levels of TFPI of ESRD patients itself, and the plasma TFPI level decreased during hemodialysis when using citrate for anticoagulation. Our study indicated that the pre-dialysis plasma level of TFPI was slightly higher in ESRD patients of the citrate group than that in controls. This may be due to the coagulation disorders in patients of the citrate group after receiving long-term hemodialysis. During hemodialysis, the anticoagulant effect of heparin is approximately 30%, mediated by the TFPI released from its endothelial stores into the circulating blood, which will lead to untoward depletion of TFPI (30,31). Repeated and continuous heparin administration will lead to excessive depletion of intravascular pools of TFPI, thereby resulting in attenuation of its contribution to the antithrombotic action of heparin (32) and protective effect on atherosclerosis. However, the results should be viewed with caution because the effect of dialysis cannot be ruled out.
The hemodialyzer membrane activates PMNs and promotes the release of PMNE (33,34). PMNE is a marker of the biological incompatibility of the dialyzer. Heparin is reported to stimulate PMNs in synergy with the stimulatory effect of dialysis membranes in vivo (35), which is similar to the PMNE changes in the LMWH group in our research. Meanwhile, the PMNE changes of the citrate group in our study reconfirmed that when eliminating the effect of heparin, hemodialysis itself also stimulated PMNs and elevated the PMNE level. PMNE was considered to reflect adequate anticoagulative activity, as when anticoagulation was inadequate, the elastase values rose consistently (36). TFPI, as the unique inhibitor of the extrinsic coagulation pathway, plays an important part in defending thrombosis during hemodialysis (37). Recently, Massberg et al. bred mice with injured carotid arteries that were deficient in PMNE and cathepsin G (Elane−/−; Ctsg−/− mice), attesting that PMNE inactivated TFPI by involving local proteolysis of TFPI and then sequentially promoted intravascular thrombus growth in vivo (12). In our study, we also found a possible correlation between the plasma level of TFPI and PMNE during a heparin-free hemodialysis session. We didn’t find such correlation in the LMWH group probably because heparin has a great influence on TFPI but a little influence on PMNE, which is mainly affected by hemodialyzer membrane. The present study suggests that when eliminating the effect of heparin on TFPI and PMNs, PMNE might play a role in anticoagulative activity through TFPI during hemodialysis.
The study, however, also bears some limitations. Although plasma levels of TF and TFPI can represent for their content in the body, alternatively spliced isoforms of TF and TFPI are differentially expressed by endothelial cells and human platelets (10,38). We only measured the plasma level of TF and TFPI, and it exists with some limitations. A prospective cohort study with a large number of hemodialysis subjects and direct biochemical research is required to confirm our results.
In conclusion, the change in the plasma TF level during HD is associated with chronic renal failure superior to HD. When eliminating the effect of heparin, hemodialysis can reduce the plasma concentration of TFPI, and PMNE might play a role in anticoagulative activity through TFPI during HD. Increasing or maintaining TFPI concentration may be a new target for anticoagulation and improving the biocompatibility of the hemodialysis membrane during hemodialysis, especially in ESRD patients with a high risk of bleeding complications.
Acknowledgments
Funding: This work was supported by a grant from the Key Program of National Natural Science Foundation (51433007-1), Research and Development of Key Technology of Wearable Artificial Kidney of Sichuan University (2016YFC1103003), Sichuan, China, and a research grant from the German Research Foundation (ZA428/10-2) to AZ. We acknowledge Dr. Pierre Delanaye for English editing and revising.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Research Ethics Committee of West China Hospital of Sichuan University (2016-188), and informed consent was obtained from all participants. The authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Hofbauer R, Moser D, Frass M, et al. Effect of anticoagulation on blood membrane interactions during hemodialysis. Kidney Int 1999;56:1578-83. [Crossref] [PubMed]
- Nseir V, Rachas A, Elias M, et al. Comparison of the AN69ST Membrane versus Citrate-Enriched Dialysate on Clotting Events during Hemodialysis without Systemic Anticoagulation. Blood Purif 2017;44:60-5. [Crossref] [PubMed]
- Lohr JW, Schwab SJ. Minimizing hemorrhagic complications in dialysis patients. J Am Soc Nephrol 1991;2:961-75. [PubMed]
- Suranyi M, Chow JS. Review: anticoagulation for haemodialysis. Nephrology (Carlton) 2010;15:386-92. [Crossref] [PubMed]
- Tolwani AJ, Wille KM. Anticoagulation for continuous renal replacement therapy. Semin Dial 2009;22:141-5. [Crossref] [PubMed]
- Schneider AG, Journois D, Rimmele T. Complications of regional citrate anticoagulation: accumulation or overload? Crit Care 2017;21:281. [Crossref] [PubMed]
- Meerarani P, Moreno PR, Cimmino G, et al. Atherothrombosis: role of tissue factor; link between diabetes, obesity and inflammation. Indian J Exp Biol 2007;45:103-10. [PubMed]
- Pawlak K, Pawlak D, Mysliwiec M. Tissue factor and urokinase-type plasminogen activator system are related to the presence of cardiovascular disease in hemodialysis patients. Thromb Res 2007;120:871-6. [Crossref] [PubMed]
- Lakbakbi S, Debrumetz A, Terryn C, et al. Tissue factor expressed by adherent cells contributes to hemodialysis-membrane thrombogenicity. Thromb Res 2016;144:218-23. [Crossref] [PubMed]
- Mast AE. Tissue Factor Pathway Inhibitor: Multiple Anticoagulant Activities for a Single Protein. Arterioscler Thromb Vasc Biol 2016;36:9-14. [Crossref] [PubMed]
- Lwaleed BA, Bass PS. Tissue factor pathway inhibitor: structure, biology and involvement in disease. J Pathol 2006;208:327-39. [Crossref] [PubMed]
- Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010;16:887-96. [Crossref] [PubMed]
- Petersen LC, Bjorn SE, Nordfang O. Effect of leukocyte proteinases on tissue factor pathway inhibitor. Thromb Haemost 1992;67:537-41. [Crossref] [PubMed]
- Lupu C, Poulsen E, Roquefeuil S, et al. Cellular effects of heparin on the production and release of tissue factor pathway inhibitor in human endothelial cells in culture. Arterioscler Thromb Vasc Biol 1999;19:2251-62. [Crossref] [PubMed]
- Bazzoni G, Beltran Nunez A, Mascellani G, et al. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. J Lab Clin Med 1993;121:268-75. [PubMed]
- Lazarowski ER, Santome JA, Behrens NH, et al. Aggregation of human neutrophils by heparin. Thromb Res 1986;41:437-46. [Crossref] [PubMed]
- Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713-35. [Crossref] [PubMed]
- Inoue A, Wada H, Takagi M, et al. Hemostatic abnormalities in patients with thrombotic complications on maintenance hemodialysis. Clin Appl Thromb Hemost 2000;6:100-3. [Crossref] [PubMed]
- Naumnik B, Borawski J, Mysliwiec M. Different effects of enoxaparin and unfractionated heparin on extrinsic blood coagulation during haemodialysis: a prospective study. Nephrol Dial Transplant 2003;18:1376-82. [Crossref] [PubMed]
- Borawski J, Naumnik B, Mysliwiec M. Tissue factor and thrombomodulin in hemodialysis patients: associations with endothelial injury, liver disease, and erythropoietin therapy. Clin Appl Thromb Hemost 2002;8:359-67. [Crossref] [PubMed]
- Zemanova P, Opatrny K, Vit L, et al. Tissue factor, its inhibitor, and the thrombogenicity of two new synthetic membranes. Artif Organs 2005;29:651-7. [Crossref] [PubMed]
- Pawlak K, Borawski J, Naumnik B, et al. Relationship between oxidative stress and extrinsic coagulation pathway in haemodialyzed patients. Thromb Res 2003;109:247-51. [Crossref] [PubMed]
- Pawlak K, Naumnik B, Brzosko S, et al. Oxidative stress - a link between endothelial injury, coagulation activation, and atherosclerosis in haemodialysis patients. Am J Nephrol 2004;24:154-61. [Crossref] [PubMed]
- Mercier E, Branger B, Vecina F, et al. Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol J 2001;2:18-25. [Crossref] [PubMed]
- Serradell M, Diaz-Ricart M, Cases A, et al. Uremic medium disturbs the hemostatic balance of cultured human endothelial cells. Thromb Haemost 2001;86:1099-105. [Crossref] [PubMed]
- Gondouin B, Cerini C, Dou L, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int 2013;84:733-44. [Crossref] [PubMed]
- Pasterk L, Lemesch S, Leber B, et al. Oxidized plasma albumin promotes platelet-endothelial crosstalk and endothelial tissue factor expression. Sci Rep 2016;6:22104. [Crossref] [PubMed]
- Kario K, Matsuo T, Yamada T, et al. Increased tissue factor pathway inhibitor levels in uremic patients on regular hemodialysis. Thromb Haemost 1994;71:275-9. [Crossref] [PubMed]
- Cella G, Vertolli U, Naso A, et al. Tissue factor pathway inhibitor (TFPI) activity in uremic patients during hemodialysis. Thromb Res 1996;81:671-7. [Crossref] [PubMed]
- Borawski J, Gozdzikiewicz J, Dubowski M, et al. Tissue factor pathway inhibitor release and depletion by sulodexide in humans. Adv Med Sci 2009;54:32-6. [Crossref] [PubMed]
- Grandone E, Chinni E, Villani M, et al. Modulation of factors involved in placental haemostasis and angiogenesis by low-molecular-weight-heparins. Arch Gynecol Obstet 2016;294:1323-9. [Crossref] [PubMed]
- Hansen JB, Sandset PM, Huseby KR, et al. Depletion of intravascular pools of tissue factor pathway inhibitor (TFPI) during repeated or continuous intravenous infusion of heparin in man. Thromb Haemost 1996;76:703-9. [Crossref] [PubMed]
- Naskalski JW, Kapusta M, Fedak D, et al. Effect of hemodialysis on acid leukocyte-type ribonuclease, alkaline ribonuclease and polymorphonuclear elastase serum levels in patients with end-stage renal disease. Nephron Clin Pract 2009;112:c248-54. [Crossref] [PubMed]
- Bos JC, Grooteman MP, van Houte AJ, et al. Low polymorphonuclear cell degranulation during citrate anticoagulation: a comparison between citrate and heparin dialysis. Nephrol Dial Transplant 1997;12:1387-93. [Crossref] [PubMed]
- Leitienne P, Fouque D, Rigal D, et al. Heparins and blood polymorphonuclear stimulation in haemodialysis: an expansion of the biocompatibility concept. Nephrol Dial Transplant 2000;15:1631-7. [Crossref] [PubMed]
- Swars H, Hafner G, Weilemann LS, et al. Acute dialysis: PMN-elastase as a new parameter for controlling individual anticoagulation with low molecular weight heparin (Fragmin). Intensive Care Med 1991;17:52-6. [Crossref] [PubMed]
- Yorioka N, Taniguchi Y, Yamashita K, et al. Tissue factor and tissue factor pathway inhibitor in hemodialysis patients. Int J Artif Organs 1998;21:699-701. [Crossref] [PubMed]
- Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation 2006;113:722-31. [Crossref] [PubMed]


