
Vitamin D-binding protein (rs4588) T/T genotype is associated with anteroseptal myocardial infarction in coronary artery disease patients
Introduction
According to the World Health Organization (WHO), cardiovascular diseases (CVD) are among leading causes of death worldwide and account for 31% of all global deaths and 47% of all deaths at the European level (1). Elevated blood pressure, smoking, diabetes and hyperlipidemia still remain the main risk factors for development of the coronary heart disease, while physical inactivity, obesity, poor nutrition and low socio-economic status are considered to be the predisposing factors. Many other factors show correlation with coronary disease including proinflammatory factors and individual genetics (2-5). French, Italians, Irish (Northern), Albanians and Indians in their scientific literature point out national paradoxes deviating from the known pathogenesis of the development of CVDs and correlations with the known risk factors; hence it is understandable that the etiology of occurrence of coronary disease has not been sufficiently researched and it is still a subject of intense investigations (6).
Amongst CVD, coronary artery disease (CAD), is denoted as a pathologic process that affects coronary arteries (7). Including acute myocardium infarct (MI), it accounts for almost half of all cardiovascular deaths as the most common cause of death in the developed world (8). The large panel of predisposing factors still fail to explain the complex pathogenesis of CAD on the molecular level. Recently, a rather old concept on the role of inflammation as a driving mechanism of systemic changes leading to CAD, has been substantiated by a growing body of novel evidence (9). For example, a high degree of correlation in key inflammatory genes was recently found between subjects with CAD and the metabolic syndrome, which is not surprising knowing that diet and lifestyle heavily underlie CAD pathogenesis (10). The importance of immune processes in CAD is also underpinned by a well-studied role of innate immunity in the atherogenesis formation of the vessel lesions, their progression and ultimate clinical presentation (11). From this angle, growing research on vitamin D role in the innate immune response, and as such a possible predisposing factor for CVD, has been conducted (12). There are two main forms of vitamin D that can be consumed from food, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol). Vitamin D3 is also synthesized in the skin by conversion of 7-dihydroxy-cholesterol via exposure to UV-B rays (13). Both vitamins D2 and D3 are hydroxylated in the liver into steroid hormones 25-hydroxyvitamin D2 and D3 [25(OH)D] that are consequently 1-alpha-hydroxylated into the active vitamin D form 1,25-dihydroxy vitamin D2 and D3 [1,25(OH)D], mainly in the kidneys with parathyroid hormone (PTH) mediation. 1,25(OH)D binds to the ubiquitous vitamin D nuclear receptors (VDR) and influences transcription of a large number of vitamin D target genes. This whole metabolic pathway has a pivotal systemic role, e.g., it is essential for calcium homeostasis, healthy growth and remodeling of bone, neuromuscular and immune functions, as well as in reduction of inflammation and antioxidant processes. It is not therefore, surprising that low circulating 25(OH)D levels were found to correlate with general morbidity and mortality (14,15), or are associated with CVD (16,17) or CAD (18). Still, supplementation with vitamin D in CVD patients in controlled clinical trials did not prove effective as expected which hampers establishing of a direct link between 25(OH)D serum levels with CVD occurrence or outcome (19). The missing link may be in the genetic background as a number of papers report on specific genetic features that account for major differences in response to vitamin D supplementation or for differences in the 25(OH)D serum levels between races or populations (20,21). In this context, vitamin D-binding protein (VDBP) gene gained particular interest due to its importance in vitamin D metabolism and transport in the body. It is a highly polymorphic gene mapped on the chromosome 4 (22) and synthesized in the liver as the main carrier of vitamin D metabolites in the body, namely 25(OH)D and 1,25-dihydroxyvitamin D. Biological effects of vitamin D on target cells may be also affected by genetic features of the VDBP gene (23). Some authors showed that genetic differences in VDBP gene cannot independently predict the prevalence and extent of CAD (24) while some showed association of the VDBP polymorphisms Thr420Lys (rs4588) and Asp416Glu (rs7041) with CAD (25). As literature data is still not conclusive on the VDBP polymorphisms role in CAD, our objective was to provide additional evidence on the association of VDBP polymorphisms (rs4588) and (rs7041) with CAD in patients after acute MI and to study possible correlations of these polymorphisms with 25(OH)D serum levels.
Methods
Patients and samples
The cross-section genotyping study included 155 subjects with CAD upon acute myocardial infarction (MI) and 104 control subjects (Table 1) at Thalassotherapia Opatija, Clinic for rehabilitation, treatment, and prevention of diseases of the heart and blood vessels, Croatia. All patients and control group were Caucasians of European descent from the population of the Primorsko Goranska-County (Croatia). The patients within the CAD group have a medical history with the diagnosis of arterial hypertension (AH) (Table S1) and are under prescribed therapy. Lower mean blood pressure value is therefore due to adequate antihypertensive therapy. The test group consisted of patients undergoing stationary cardiologic rehabilitation program at Thalassotherapia Opatija after acute MI. In the control group higher mean blood pressure value was calculated than in the CAD group, but the value is still below the cut-off value for AH diagnosis (<140/90 mmHg). All patients were subjected to peracute coronary revascularization. The control group comprised healthy subjects of adequate age and gender, undergoing routine systematic examination. The research was approved by the Ethics Committee of Thalassotherapia Opatija and the Committee for Ethical Issues of the Faculty of Medicine of the University of Rijeka, Croatia. Data on age and sex, early illnesses and data on risk factors and habits (smoking, AH, diabetes, family anamnesis) were collected for all subjects (Table S1). The type of acute MI was determined by the electrocardiographic features (the acute MI with or without the elevation of ST segment), coronarography findings and invasive therapy. The excluding criteria for the study were: proven existence of malignant disease, kidney failure, primary hyperparathyroidism and substitute therapy for calcium and vitamin D. All subjects signed the informed consent pursuant to the Helsinki Declaration, approved by the Ethics Committee of Thalassotherapia Opatija and the Committee for Ethical Issues of the Faculty of Medicine. Detailed procedures used for clinical evaluation of patients are described in Supplemental file 1.

Full table

Full table
Genomic DNA extraction and genetic analysis
Genomic DNA was extracted from 200 µL peripheral blood using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany), on a QIAcube robotic workstation (Qiagen), according to the manufacturer’s manual. DNA quality and concentration were measured using a BioDrop spectrophotometer. Genotyping of VDBP gene polymorphisms (rs4588) and (rs7041) was performed with TaqMan Pre-designed SNP Genotyping Assay on an Applied Biosystems 7500 fast real-time PCR machine according to manufactures recommendation. The PCR cycling conditions were 95 °C for 10 min, followed by 45 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 minute. Collected results were analyzed with allelic discrimination analysis software available on Applied Biosystems 7500 fast PCR instrument.
Vitamin D serum levels measurement
Serum samples (after an overnight fasting) were collected for 118 CAD patients after MI and 70 control subjects and stored at −80 °C. Levels of 25(OH)D2 and 25(OH)D3 were measured by use of the ACQUITY UPLC I-Class and Xevo TQD (Milford, Massachusetts, USA). For experiment creation and results analysis, MassLynx software (Waters, USA) was used, while the UPLC parameters were controlled through ACQUITY UPLC console. Solid phase extraction and liquid chromatography, tandem mass spectrometry (LC-MS/MS) procedures are described in the Supplemental file 1.
Statistics
Collected data were statistically evaluated using data analysis software system Dell Statistica, version 12. Dell Inc. (2015). Categorical variables are presented with frequencies or percentages and comparisons done using Pearson chi-square (χ2) test. The association of categorical variables was expressed as odds ratio (OR) with a 95% confidence interval (CI). Normally distributed continuous variables (distribution tested with Kolmogorov-Smirnov test) are presented as means ± standard deviation. Comparisons of values between two groups were made using t-test or between multiple groups using analysis of variance (ANOVA test), with post-hoc Scheffe testing. Factor covariance was examined using analysis of covariance (ANOVA). Statistical significance was determined at the level of 0.05. Univariate and multivariate logistic regression analysis was employed to determine the predicting value of parameters for discrimination between the two groups and adjust for differences. Obtained models were confirmed by receiver operator characteristic (ROC) analysis.
Results
The herein analyzed patient cohort comprised a total of 124 CAD patients (80.0%) upon acute MI with the ST segment elevation (STEMI) and 31 patients (20.0%) with acute MI without ST segment elevation (NSTEMI). The most common localization of the MI was the anteroseptal wall of the left ventricle (61 subjects, 39.4%) and inferoposterior localization (61 subjects, 39.4%) while 5 data subjects (3.2%) had the lateral localization.
For further statistical evaluation, patients were subdivided into subgroups comprising STEMI patients (STEMI CAD group) and NSTEMI patients (NSTEMI CAD group). Evaluation of obtained results was also done in accordance with the spatial MI presentation, anteroseptal MI (ASMI CAD), inferior wall MI and lateral wall MI. No statistically relevant differences were observed between the STEMI CAD and NSTEMI CAD groups in comparison with control for the total vitamin D and 25(OH)D3 serum levels (ANOVA, P>0.05, Table S2). Moreover, the measured values for total vitamin D in the serum of all groups were within the suggested range 50–125 nmol/L (26). Statistically higher levels of 25(OH)D2, probably due to unreported vitamin D supplementation, were observed in the CAD group (t-test, t=2.61, P=0.01) in comparison with control. Considering that vitamin D3 biological relevance in humans is higher than those of vitamin D2 this result does not suggest a direct clinical implication for the CAD in this case (27,28).

Full table
Genotyping data showed that the VDBP (rs4588) genotype T/T was statistically marginally more prevalent (χ2 test: P=0.08) in the CAD group in comparison with control (Table S3). No statistically relevant differences were observed for the VDBP (rs7041) genotypes between CAD and control. Moreover, analysis of allele frequencies and genotypes for VDBP (rs4588) and (rs7041) polymorphisms in the CAD group in comparison with controls showed no statistical relevance (Tables S4,S5). However, when the analysis of the VDBP (rs4588) genotype was performed according to the clinical presentation of the MI, the VDBP (rs4588) T/T genotype was statistically more prevalent (P=0.034) in the ASMI CAD group in comparison with control (Table 2). Statistical comparison between other groups yielded no statistically relevant data. However, in the recessive model the T/T genotype for VDBP (rs4588) was associated with a 2.94-fold significantly higher risk (95% CI: 1.13–7.66, P=0.023) for anteroseptal MI (Table 3).
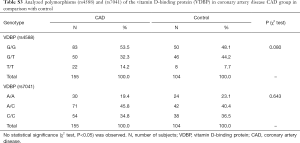
Full table

Full table
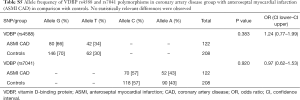
Full table

Full table
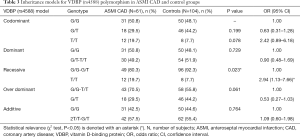
Full table
Due to significant differences observed between CAD and control groups related to the gender, AH diagnosis and smoking, additional interaction analyses were performed. Significant interactions were found between VDBP (rs4588) and gender where male had more prevalently the G/G variant while woman had more prevalently the G/T variant (Table S6). Moreover, for AH, a significant interaction was observed only for AH and the group while AH correlation with VDBP (rs4588) was not significant (data not shown). At last, smokers were less represented by the VDBP (rs4588) G/G variant and more by the VDBP (rs4588) T/T variant (Table S7). As smoking is a known CAD risk factor, a statistical correlation between the VDBP(rs4588) T/T genotype and CAD patients who are smokers compared to non-smoking controls was interesting (χ2=8.59, P=0.014 and recessive model: χ2=7.69, P=0.005; OR =4.51; 95% Cl: 1.44–14.14) (Table 4) suggesting that VDBP(rs4588) T/T genotype may be one of the risk factors for development of CAD in smokers.

Full table
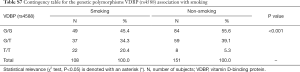
Full table

Full table
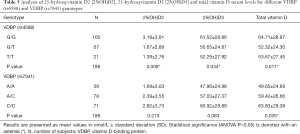
The VDBP (rs4588) genotype was analysed in comparison with the 25(OH)D2, 25(OH)D3 and total vitamin D serum levels in both CAD patients and controls (Table 5). Genetic variation in VDBP (rs4588) influences 25(OH)D serum levels upon vitamin D3 and not vitamin D2 supplementation (29) and the herein employed LC-MS/MS method was therefore used for measurement of corresponding 25(OH)D forms and total vitamin D levels in the serum (30,31). VDBP (rs4588) genotype G/G presence correlated with higher levels of 25(OH)D2 (ANOVA, P=0.008). Similarly, the VDBP (rs4588) genotype G/G presence correlated with higher 25(OH)D3 levels (ANOVA; P=0.034). At last, the VDBP (rs4588) genotype G/G presence correlated with higher levels of total vitamin D (ANOVA; P=0.011). Marginal statistical correlation for VDBP (rs7041) C/C genotype presence with higher levels of total vitamin D was also observed (ANOVA; P=0.035). For 25(OH)D3 and VDBP (rs4588) (analysis of covariance, F=4.62, P=0.033) as well as total vitamin D and VDBP (rs4588) (analysis of covariance, F=4.70, P=0.031) the age was a significant covariate in the CAD group with the VDBP (rs4588) G/T genotype where levels of vitamin D and age were slightly negatively correlated with 25(OH)D3 and total vitamin D serum levels.
Full table
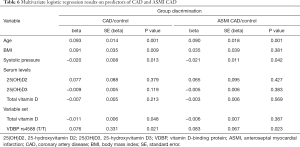
Finally, logistic regression analysis was performed to analyse blood pressure, smoking and body mass index (BMI) confounding on vitamin D levels and VDBP (rs4588) genotype in CAD and ASMI CAD groups (Table 6). Although vitamin D levels (D2, D3 and total D vitamin level) did not show predicting value for both CAD and ASMI CAD group discrimination from control, the multivariate logistic regression modelling enabled association of total vitamin D level and VDBP (rs4588 T/T genotype with CAD and ASMI CAD occurrence (Table 6). Logistic regression (adjusted for BMI, smoking and systolic pressure), showed lower levels of total vitamin D with VDBP (rs4588) T/T genotype discriminate CAD from healthy individuals [model P=0.046, R2 =0.057, ROC area under the curve (AUC) =0.624, P=0.037]. The same goes for ASMI CAD distinction from control group (model P=0.037, R2 =0.047, ROC AUC =0.625, P=0.034).
Full table
Discussion
Vitamin D plays a pivotal role in the organism and it regulates the function of 3% of the human genome (32). Although 1,25(OH)D is the active form of this vitamin, the serum quantity of 25(OH)D is clinically relevant (33). Recent studies brought scientific evidence on connection of 25(OH)D serum levels with beneficial effects on fibrinolysis, lipid profile, thrombogenicity, regeneration of the endothelium and smooth muscle cell growth (34) but also with hypertension and metabolic syndrome (35). Also, low values of circulating 25(OH)D are associated with an increased risk of CVD as well and with the corresponding mortality (14,36). In the herein presented study, the levels of total vitamin D in the serum were normal in all tested subjects which was partially expected as in the geographical area (Primorsko-Goranska County) where the tested subjects were recruited, the number of sunny days is abundant and the population has a wider approach to the Mediterranean type of diet. However, the levels of 25(OH)D in the serum of tested subjects were statistically significantly different according to the VDBP genotype which is expected as more than 85% of 25(OH)D in the serum is bound to VDBP (37). The herein analyzed VDBP polymorphisms (rs4588) and (rs7041) account for about 80% of the variability in 25(OH)D serum levels (22,38) and the VDBP (rs4588) genotype G/G presence correlated with higher levels of 25(OH)D2, higher levels of 25(OH)D3 and higher levels of total vitamin D in the serum. Observed genotype-phenotype correlations were not associated with MI. However, VDBP (rs4588) T/T genotype was statistically marginally more prevalent (χ2 test: P=0.08) in the MI CAD group in comparison with control. Interestingly, although D2, D3 and total D vitamin levels did not show predicting value for both CAD and ASMI CAD group discrimination from control, the multivariate logistic regression modelling enabled association of total vitamin D level and VDBP (rs4588) T/T genotype with CAD and ASMI CAD occurrence and lower levels of total vitamin D with VDBP (rs4588) T/T genotype discriminate CAD from healthy individuals. The same goes for ASMI CAD distinction from control group. This result should be further studied on a larger cohort to derive conclusive results. Indeed, Stakisaitis et al. (38) showed a correlation of VDBP polymorphism (rs7041) C allele with increased risk of CAD. In another study on an Iranian cohort, a significant association of VDBP (rs7041) G/G genotype and VDBP (rs4588) C/A genotype and allele A with CAD has been also found (25). These data are not in concordance with a large Atherosclerosis Risk in Communities (ARIC) study were no impact of the VDBP polymorphisms on CAD was observed either for black or white population (20). Similarly, Daffara et al. (23) studied a large cohort of CAD patients undergoing coronary angiography and established no correlation of the VDBP (rs4588) and (rs7041) polymorphisms with CAD. Interestingly, in all above mentioned studies the levels of 25(OH)D were assessed as deficiency in majority of tested subjects. As 25(OH)D levels in our study were normal, we analyzed the VDBP (rs4588) genotype according to the clinical presentation of the MI. The VDBP (rs4588) T/T genotype was statistically more prevalent (χ2 test: P=0.034) in the ASMI CAD group in comparison with control. Nevertheless, VDBP (rs4588) T/T genotype showed to be generally more prevalent in smokers, and ASMI CAD were also more prevalent in smokers. Moreover, T/T genotype was overrepresented in smoking CAD patients’ cohort in comparison with non-smoking controls. Even though frequencies of alleles between ASMI CAD group and control for VDBP (rs4588) were not statistically relevant, in the recessive model the T/T genotype for VDBP (rs4588) was found to be associated with a 2.94-fold significantly higher risk (95% CI: 1.13–7.66, P=0.023) for the anteroseptal MI. While it has been speculated that vitamin D deficiency may be correlated with acute MI (39)] and our study provided n evidence for this assumption, a role of T/T VDBP (rs4588) genotype has not been previously described for this specific group of patients.
It should be emphasized that the major limitation of the presented study may be in a rather small number of the samples. Still, the power calculated according to the allele frequency in the general population, hypothetical frequency of the CAD with the error level type I as 0.05 and power of analysis 80%, showed that the minimal sample size for rs4588 is 132 patients and for rs731236 116 patients, which is met in the presented study. Observed differences in the gender, AH and smoking may add bias to the results as well.
In conclusion, obtained data speak in favor to the VDBP (rs4588) T/T genotype as a susceptibility factor for anteroseptal MI where the same genotype showed to be generally more prevalent in smokers, and anteroseptal MI was also more prevalent in smokers. Larger prospective studies should be conducted to better elucidate the association of this specific VDBP genotype in correlation with smoking as susceptibility factor for anteroseptal MI.
Supplementary
Supplementary file 1
Subjects and procedures used for clinical evaluation of patients
A total of 124 patients (80.0%) had acute myocardial infarction with the ST segment elevation (STEMI), while 31 patients (20.0%) had acute myocardial infarction without STEMI (NSTEMI). Ninety-four (60.6%) of the data subjects had been subjected to percutaneous coronary intervention on the anterior descending branch of the left coronary artery. Percutaneous coronary intervention on the circumflexed branch of the left coronary artery was performed in 25 data subjects (16.1%), while in 43 data subjects (27.7%) it was performed on the right coronary artery. One hundred and seventeen patients (75.5%) of the CAD group have suffered from arterial hypertension (AH), 122 CAD subjects (78.7%) have suffered from hyperlipoproteinemia, 29 CAD subjects (18.7%) have suffered from diabetes, 5 CAD subjects (3.2%) have suffered from chronic obstructive pulmonary disease (COPD). A total number of 86 data subjects (55.5%) were smokers and 93 CAD subjects (60.0%) had a positive family history of cardiovascular diseases.
A statistically significant difference between CAD and control group was observed in gender distribution (81.9% male in CAD group vs. 65.4% male in control group). Statistically significant difference was also observed between CAD and control group in age distribution (58 vs. 47 years, P<0.001). There was also a difference in the body mass index (28.5 vs. 27.2 kg/m2, P=0.017) without clinical significance as both values were in the overweight range. While predisposing factors including hyperlipoproteinemia, COPD prevalence and positive family history of cardiovascular diseases showed no differences between groups, statistically significant difference between groups was observed in prevalence of AH (75.5% vs. 38.5%, P<0.001), diabetes mellitus (18.7% vs. 8.7%, P=0.025) and smoking (55.5% vs. 21.2%, P<0.001) (Table S1).
Blood samples of the affected subjects were taken between 7 and 8 am, the second day after the admission to rehabilitation treatment. Serum levels of vitamin D {25-hydroxyvitamin D [25(OH)D]} were determined by the mass spectrometry (Xevo TQ-S, Waters corporation, Milford USA), natriuretic peptide (BNP), high sensitive C-reactive protein (HsCRP) and other routine laboratory findings [complete blood count (CBC), plasma glucose levels, urea, creatinine, sodium, potassium, calcium aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP); bilirubin, cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), thyroid-stimulating hormone (TSH)]. Erythrocyte sedimentation was defined by Westergreen method (Becton Dickinson, Franklin Lakes, NJ, USA). Haemoglobin was defined via “Sulfolyser oxidizing method”, erythrocytes and hematocrit via “Hydro Dynamic Focusing method” on haematological analyzer (Haematology analyser XS-1000i, Sysmex, Kobe, Japan). Leukocytes, neutrophils, eosinophils, basophils, lymphocytes and monocytes were determined by the flow cytometry with the help of/means semiconductor laser on a hematologic analyzer (Haematology analyser XS-1000i, Sysmex, Kobe, Japan). Mean cell volume (MCV), mean cell hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) were calculated on the bases of the data obtained on the hematological analyzer. Bilirubin, AST, ALT, glutamyl transferase (GT), Na+, K+, urea, creatinine, high sensitivity C-reactive protein (hsCRP), cholesterol, triglycerides, HDL, LDL, TSH and urates were determined by photometric method using Dimension Xpand [Siemens Healthcare Diagnostics, Newark (DE), USA]. All the laboratory parameters were determined in the laboratory of Specialized hospital for cardiac, pulmonary and rheumatic diseases “Thalassotherapia Opatija”. During this research, the following anthropometric measurements were taken (all by using the same devices): height in meters (m), weight in kilograms (kg) by using manual scale. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in persons while sitting, after taking a rest for at least 15 minutes. Body mass index (BMI) is calculated as weight (kg)/height (m2). Ergospirometry testing was performed on a bicycle ergometer with a gradual increase in load (Cortex Metamax) and it included the following phases: start load of 50 watt in duration of 3 minutes up to 60 cycles per minute, every 3-minute increase of load for 25 watts. The criteria for achieving maximum capacity were one or more of the following: Achieving Plato VO2, maximum heart frequency greater than 90% of the anticipated values for that age (220 years), respiratory exchange ratio (RER) more than 1/15 (although RER values are not indicatively accurate for the maximum capacity). The data are automatically processed by using the standard formula (based on the reference values) and are presented as descriptive data. The measured variables include: VO2 (L/min), VCO2 (L/min), VO2/kg (mL/min/kg), load (W/min), heart rate (HR) (b/min), heart rate recovery (HRR) (b/min) (L/min), EQO2, EQCO2, minute ventilation (VE) (L/min), breathing rate (BR) (%), blood flow (BF) (L/min). All echocardiograph image acquisitions were done for all the participants at the beginning of the rehabilitation process. The tests were conducted by the examiner having 8 years of experience, who was unfamiliar with the study protocol and characteristics of the patients. Echocardiograph data were obtained via ultrasound device of the Vivid E9 system (GE Vingmed Ultrasound AS, Horten, Norway) equipped with a single 2D 3.5-MHz transducer (M5S-D), one 3D 3.5-MHz transducer (4C-D)—analytical software for monitoring peaks and 1 workstation for background processing (EchoPAC BT 11.1.0, GE Medical System, Horten, Norway). All subjects were connected to the electrocardiogram (ECG) and viewed in their left-hand side position. First the conventional echocardiography was performed for the purpose of the observation of cardiac structures from parasternal long and short axes and apical display of 4 chambers. Early and late peak diastolic flow rates (E-wave and A-wave) were measured and then the E/A proportion were calculated. Then the 3D transducer was used to obtain a clear LV endocardium image with the apical 4-chamber view in 4D mode. Patients were asked to keep their breath at the end. Imaging allows a sector with depths of 30° and a width of 100° in real time. 3D dynamic left ventricular (LV) images in the acquisition of full volume are collected and stored. The frame rate of the volumetric image was 25 to 35 frames per second. After the software has identified the borders of endocardial and epicardial lines for the entire 3D data set, the left ventricular model, left ventricular end-diastolic volume (LVEDV), left ventricular end systolic volume (LVESV), cardiac output (CO), stroke volume (SV), and the left ventricle ejection fraction (LVEF) are generated.
Solid phase extraction (SPE) procedure and liquid chromatography, tandem mass spectrometry (LC-MS/MS)
For SPE, methanol and water (Honeywell, Morris Plains, New Jersey, USA) were used for preparation of working solutions as follows: 80% methanol/20% isopropyl alcohol (IPA) (v/v) (Sigma-Aldrich, St. Louis, Missouri, USA), 60% methanol (aq.), 5% methanol (aq.), 95% methanol/5% IPA (v/v). Zinc sulfate (0.2 M), 2 mM ammonium acetate/0.1% formic acid (v/v) (aq.), 2 mM ammonium acetate/0.1% formic acid (v/v) in methanol were purchased from (Sigma-Aldrich, St. Louis, Missouri, USA). Briefly, for SPE 20 µL of internal standard mix was added into 150 µL plasma samples aliquots and vortexed briefly. One hundred and fifty µL of zinc sulphate (0.2 M) was added to the samples followed by addition of 600 µL of 100% methanol. Samples were then centrifuged 10 minutes at 14,000 rpm. After SPE, supernatant containing 25(OH)D2 and 25(OH)D3 was obtained and collected for each sample. Samples were additionally eluted by use of OASIS® HLB µElution plate 30 µm (Waters, Milford, Massachusetts, USA) for the final protein clean-up. Calibrator mix 25(OH)D3 and 25(OH)D2 solutions (Sigma-Aldrich, St. Louis, Missouri, USA) of known concentrations were used to assess the linearity of the measurement. Quality control (QC) mix of 25(OH)D3 and 25(OH)D2 solutions ClinChek® (LGC, Teddington, UK) were used as measurement quality assurance. Internal standard (250 ng/mL) was the referent solution of vitamin D2 (triply deuterated) and D3 (doubly deuterated) (Waters, Milford, Massachusetts, USA), these were added to each sample, QCs and calibrators, relative to which all 25(OH)D2 and 25(OH)D3 and concentrations were measured (internal control is to account for possible sample loss during preparation). UPLC settings included BEH-phenyl column (2.1×50 mm, 1.7 µm) (Waters, Milford, Massachusetts, USA), used to separate the OH vitamins D from lipids, mobile phase A containing 2 mM ammonium acetate in 0.1% formic acid (aq.), mobile phase B consisted of 2 mM ammonium acetate in 0.1% formic acid (in methanol), gradient elution (0.45 mL/min flow, 4.2 min run time, 10 µL injection volume, 45 °C column temperature, 10 °C sample temperature, weak wash solution containing 60%/40% (v/v) methanol/water and strong wash solution containing 100% methanol). The mass spectrometer settings were set on electrospray ionization positive mode, 1.5 kV capillary voltage, 450 °C desolvation temperature, 1,100 L/h desolvation gas flow, 150 L/h cone gas, 150 °C source temperature, 5.0 µL/min sample flow and multiple reaction monitoring acquisition mode (MRM).
Acknowledgments
We greatly acknowledge the help of the technician Mr. Marko Filipović and Mr. John Hopkins, University of Rijeka in vitamin D serum measurements and Miss Ida Linić in help during establishment of the genotyping protocol. We acknowledge the University of Rijeka project “Research Infrastructure for Campus-based Laboratories at University of Rijeka”, co-financed by European Regional Development Fund (ERDF).
Funding: This research was funded through University of Rijeka research grant uniri-biomed-18-133 (No. 1277).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was approved by the Ethics Committee of Thalassotherapia Opatija (No. 01-438/2014) and the Committee for Ethical Issues of the Faculty of Medicine of the University of Rijeka, Croatia (Code: 643-03/18-01/38, Re. no.: 2170-15-18-1). All subjects signed the informed consent pursuant to the Helsinki Declaration.
References
- Cardiovascular disease. accessed on 7th January 2018. Available online: http://www.who.int/cardiovascular_diseases/about_cvd/en/
- George R, Sivadasanpillai H, Jayakumari N, et al. Circulating Thrombotic Risk Factors in Young Patients with Coronary Artery Disease Who Are on Statins and Anti-platelet Drugs. Indian J Clin Biochem 2016;31:302-9. [Crossref] [PubMed]
- Mangge H, Becker K, Fuchs D, et al. Antioxidants, inflammation and cardiovascular disease. World J Cardiol 2014;6:462-77. [Crossref] [PubMed]
- Roberts R, Stewart AFR. 9p21 and the Genetic Revolution for Coronary Artery Disease. Clin Chem 2012;58:104-12. [Crossref] [PubMed]
- Yancy WS Jr, Westman EC, French PA, et al. Diets and clinical coronary events: the truth is out there. Circulation 2003;107:10-6. [Crossref] [PubMed]
- Sanchis-Gomar F, Perez-Quilis C, Leischik C, et al. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256-68. [Crossref] [PubMed]
- Cassar A, Holmes DR, Rihal CS, et al. Chronic Coronary Artery Disease: Diagnosis and Management. Mayo Clin Proc 2009;84:1130-46. [Crossref] [PubMed]
- Nair J, Shanker J, Jambunathan S, et al. Expression analysis of leukotriene-inflammatory gene interaction network in patients with coronary artery disease. J Atheroscler Thromb 2014;21:329-45. [Crossref] [PubMed]
- Nair J, Kakkar VV, Shanker J. Comparative analysis of inflammatory gene expression levels in metabolic syndrome & coronary artery disease. Indian J Med Res 2017;145:777-85. [Crossref] [PubMed]
- Libby P. History of Discovery: Inflammation in Atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045-51. [Crossref] [PubMed]
- Kundu R, Theodoraki A, Haas CT, et al. Cell-type-specific modulation of innate immune signalling by vitamin D in human mononuclear phagocytes. Immunology 2017;150:55-63. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Brøndum-Jacobsen P, Benn M, Tybjaerg-Hansen A, et al. 25-Hydroxyvitamin D concentrations and risk of venous thromboembolism in the general population with 18791 participants. J Thromb Haemost 2013;11:423-31. [Crossref] [PubMed]
- Schöttker B, Jorde R, Peasey A, et al. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014;348:g3656. [Crossref] [PubMed]
- Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke 2006;37:243-5. [Crossref] [PubMed]
- Pekkanen MP, Ukkola O, Hedberg P, et al. Serum 25-hydroxyvitamin D is associated with major cardiovascular risk factors and cardiac structure and function in patients with coronary artery disease. Nutr Metab Cardiovasc Dis 2015;25:471-8. [Crossref] [PubMed]
- Aggarwal R, Akhthar T, Jain SK. Coronary artery disease and its association with Vitamin D deficiency. J Midlife Health 2016;7:56-60. [Crossref] [PubMed]
- Pittas AG, Chung M, Trikalinos T, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med 2010;152:307-14. [Crossref] [PubMed]
- Michos ED, Misialek JR, Selvin E, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis 2015;241:12-7. [Crossref] [PubMed]
- Michos ED, Reis JP, Post WS, et al. 25-hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition 2012;28:367-71. [Crossref] [PubMed]
- Cleve H, Constans J. The mutants of the vitamin-D-binding protein: more than 120 variants of the GC/DBP system. Vox Sang 1988;54:215-25. [Crossref] [PubMed]
- Speeckaert M, Huang G, Delanghe JR, et al. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism Clin Chim Acta 2006;372:33-42. [Crossref] [PubMed]
- Daffara V, Verdoia M, Rolla R, et al. Impact of polymorphism rs7041 and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr Metab Cardiovasc Dis 2017;27:775-83. [Crossref] [PubMed]
- Tarighi S, Najafi M, Hossein-Nezhad A, et al. Association Between Two Common Polymorphisms of Vitamin D Binding Protein and the Risk of Coronary Artery Disease: A Case-control Study. J Med Biochem 2017;36:349-57. [Crossref] [PubMed]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
- Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 2004;89:5387-91. [Crossref] [PubMed]
- Trang HM, Cole DE, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 1998;68:854-8. [Crossref] [PubMed]
- Nimitphong H, Saetug S, Chanprasertyotin S, et al. Changes in circulating 25-hydroxyvitamin D according to vitamin D binding protein genotypes after vitamin D3 or D2 supplementation. Nutr J 2013;12:39-46. [Crossref] [PubMed]
- Binkley N, Krueger D, Cowgill CS, et al. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 2004;89:3152-7. [Crossref] [PubMed]
- van den Ouweland JM, Vogeser M, Bächer S. Vitamin D and metabolites measurement by tandem mass spectrometry. Rev Endocr Metab Disord 2013;14:159-84. [Crossref] [PubMed]
- Rowling MJ, Kemmis CM, Taffany DA, et al. Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxy-cholecalciferol actions in human mammary cells. J Nutr 2006;136:2754-9. [Crossref] [PubMed]
- Pike JW, Zella LA, Meyer MB, et al. Molecular actions of 1,25-dihydroxyvitamin D3 on genes involved in calcium homeostasis. J Bone Miner Res 2007;22 Suppl 2:V16-9. [Crossref] [PubMed]
- Ku YC, Liu ME, Ku CS, et al. Relationship between vitamin D deficiency and cardiovascular disease. World J Cardiol 2013;5:337-46. [Crossref] [PubMed]
- Al Mheid I, Patel RS, Tangpricha V, et al. Vitamin D and cardiovascular disease: is the evidence solid? Eur Heart J 2013;34:3691-8. [Crossref] [PubMed]
- Brøndum-Jacobsen P, Benn M, Jensen GB, et al. 25-hydroxyvitamin D levels and risk of ischemic heart disease, myocardial infarction, and early death population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol 2012;32:2794-802. [Crossref] [PubMed]
- Ying HQ, Sun HL, He BS, et al. Circulating vitamin D binding protein, total, free and bioavailable 25-hydroxyvitamin D and risk of colorectal cancer. Sci Rep 2015;5:7956. [Crossref] [PubMed]
- Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med 2013;369:1991-2000. [Crossref] [PubMed]
- Stakisaitis D, Lesauskaitė V, Girdauskaitė M, et al. Investigation of Vitamin D-Binding Protein Polymorphism Impact on Coronary Artery Disease and Relationship with Longevity: Own Data and a Review. Int J Endocrinol 2016;2016:8347379. [Crossref] [PubMed]
- Milazzo V, De Metrio M, Cosentino N, et al. Vitamin D and acute myocardial infarction. World J Cardiol 2017;9:14-20. [Crossref] [PubMed]


