Cardiac MRI-guided interventional occlusion of ventricular septal rupture in a patient with cobalt alloy stent
Introduction
Ventricular septal rupture (VSR) is an uncommon but catastrophic complication from an acute myocardial infarction (AMI) and requires surgical repair (1-3). The location and size of VSR are commonly assessed using echocardiography and ventriculography (4). However, echocardiography has a limited field of visualization and is subject to operator bias. Ventriculography can visualize VSR, but there are still some limitations in displaying the three-dimensional structure of the rupture accurately. Previous studies have shown that cardiac magnetic resonance imaging (MRI) does provide accurate information on VSR location and size and can be used to guide surgical repairs (5-7). However, MRI is susceptible to interference by metal objects and thus is rarely used in patients with metallic implants.
Here, we report a VSR repair using MRI examination after a few days of coronary stent implantation.
Case presentation
A 68-year-old man presented with chest pain for 3 days. Electrocardiogram tests revealed that the patient had a sinus rhythm, abnormal Q wave on V1-V3, and an arched ST segment. Transthoracic echocardiography showed mild mitral and tricuspid regurgitation, and impaired left ventricular systolic function. Also, the patient had a calculated ejection fraction (EF) of 0.45. Coronary angiography demonstrated mild stenosis of the proximal segment and subtotal occlusion of the middle segment of the left anterior descending coronary artery. A Firebird 2 rapamycin-eluting coronary CoCr stent (3.0 mm × 33 mm; MicroPort, Shanghai, China) was implanted after balloon dilation.
On postoperative day 3, a harsh grade IV/V systolic ejection murmur was noticed upon auscultation along the left border of the sternum from the 3rd and 4th intercostal space to the cardiac apex. Blood pressure was 89/51 mmHg. Transthoracic echocardiography revealed massive bilateral pleural effusion. A thoracentesis was performed, with a drainage volume of 800 mL. Routine and biochemical tests indicated that the drained pleural fluid was exudate.
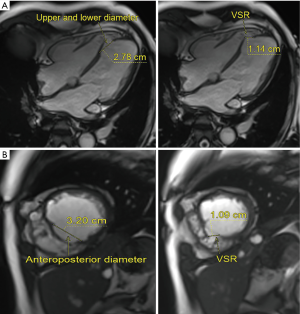
A transthoracic echocardiography showed a VSR near the apex and concurrent ventricular aneurysm. The VSR on echocardiography was 7 mm in diameter, EF was 0.47, and the inner diameter of the left ventricle was 45 mm. Scans with 3.0-T MRI (Siemens) confirmed the VSR and left-to-right shunt (Figure 1) with a VSR diameter of 11 mm. The anteroposterior and superoinferior diameter of the membranous septum was approximately 32 and 27.8 mm, respectively. The ventricular aneurysm was in the medioanterior interventricular septum and lower ventricular wall.
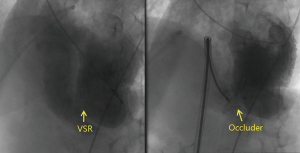
Four weeks later, interventional therapy was performed to repair the VSR. The VSR was 9.2 mm in diameter upon intraoperative left ventriculography. A 22-mm muscular interventricular septum occluder (Shanghai Shape Memory Alloy Co., Ltd., Shanghai, China) was chosen based on MRI. The procedure was successful on the first attempt (Figure 2). After the therapy, blood pressure rapidly normalized. Pleural effusion markedly reduced in volume. Repeat echocardiography on day 3 revealed no shunt, and the patient was discharged.
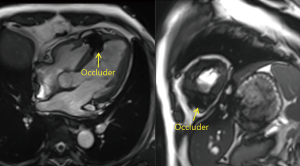
At the 4-month follow-up visit, a cardiac MRI revealed there to be a complete occlusion of the VSR. The border of the umbrella occluder was secured in the normal cardiac tissues (Figure 3). At the last follow-up visit (2 years later), the patient was eventless.
iMDT discussion
VSR is a catastrophic complication of AMI and typically occurs in the anterior or apical portion of the ventricular septum (8). Surgical repair is the only method to restore structural integrity and hemodynamics in patients with VSR (9). Immediate VSR repair is associated with a high postoperative mortality (8,10). VSR repair is now increasingly conducted with a 3- to 4-week delay to allow scarring of the surrounding tissue thus providing a firmer anchoring of the suture and patch material. Recently, interventional occlusion has become the gold standard for VSR repair (11).
Echocardiography and left ventriculography are the most commonly used methods to assess the location and size of VSR. The diameter of VSR in the current case was 7 mm upon echocardiography. Considering the limitation of echocardiography, we conducted a cardiac MRI despite the presence of a newly implanted metal stent. The VSR diameter revealed by MRI was significantly larger at 11 mm. Cardiac MRI also provided detailed information on the anatomy of the membranous septum. Such information offers valuable guidance for anchoring the occluder in a proper location to minimize the risk of enlarging the rupture. The delineation of the anatomic dimensions of the interventricular septum allows for a selection of an appropriately sized occluder that extends beyond the anteroposterior and superoinferior diameter of the membranous septum.
The advantage of MRI includes high spatial resolution and tissue specificity (12). MRI is also more versatile than echocardiography and left ventriculography. Therefore, imaging can be conducted at any plane with a sufficient visual field. A previous study suggested that a 3.0T MRI is safe in patients with a cobalt alloy drug-eluting stent (13). Successful use of cardiac MRI in the current case provided support, albeit anecdotal, to such a notion.
In summary, cardiac MRI may be conducted to provide a more accurate assessment of the location and size of VSR in patients with cobalt alloy stent.
The current Expert Consensus Statements recommend the optimal operation opportunity of occlusion for the VSR in patients with AMI: perform an occlusion surgery at the time of 4 weeks after VSR. However, in clinical practice, not all patients can wait 4 weeks to undergo the interventional therapy. In these cases, can we advance the therapy? What is the proper time to perform the interventional therapy?
Expert opinion 1: Dr. Irbaz Hameed and Dr. Arash Salemi
The optimal timing for surgical repair of VSR remains controversial. The perceived benefit of delayed surgery stems from the rejuvenation of friable tissue allowing it to become well-differentiated from surrounding healthy tissue, thus facilitating repair. In this scenario, close follow-up in the intensive care unit may be considered to enable tissue healing and promote chances of definitive repair. While the 2013 American College of Cardiology and American Heart Association guidelines recommend emergent surgical repair regardless of patients’ hemodynamic status (14), the 2017 European Society of Cardiology guidelines promote delayed elective repair in patients initially responding to aggressive conservative management (15). The timing of surgery must be individualized to patients. In stable patients with preserved end-organ function, early timing of surgical correction must be considered due to the possibility of unpredictable hemodynamic compromise. Surgery can be delayed in these patients if surgical anatomy is complex and there is uncertainty regarding tissue fragility. In this situation, close treatment in the intensive care unit should be considered to promote healing and definitive tissue repair. The watch and wait approach in these patients may also be suitable in scenarios of dual antiplatelet therapy and significant platelet inhibition (16).
Expert opinion 2: Dr. Gianfranco Butera
The Society of Thoracic Surgeons National Databases showed that a longer interval between diagnosis of MI and surgical repair (>21 days) is associated with lower operative mortality (17). However, recent European guidelines reported that there is no true consensus on the timing of intervention. In fact, while an early intervention seems to have a 20% to 40% mortality rate and a high risk of recurrent ventricular rupture, on the other side, a late closure treatment is associated with the risk of ventricular septal defect (VSD) extension and death while surgery is being awaited (15). Finally, Omar et al. found a lower mortality in patients undergoing post-myocardial infarction VSD repair 2 weeks after MI diagnosis (18).
In conclusion, it is clear that higher percentages of survival are associated with occlusion 3–4 weeks after myocardial infarction. However, sometimes the patient is not stable enough to wait and the decision should be made to anticipate the timing of the procedure.
Expert opinion 3: Dr. Evan J. Zucker
As noted in the authors’ discussion, a waiting period helps to ensure the presence of scar/granulation tissue so that surgical or implanted devices will be more likely to remain in the desired location. However, on an individual patient basis (e.g., hemodynamic instability), the risk of delaying VSR repair may be greater than the risk of the procedure or complications from the procedure. These risks should be discussed with the patient so that an informed decision can be made in the absence of abundant evidence. For example, early repair may be reasonable, with the expectation that a reoperation may be required. As new devices are developed, the optimal waiting period before repair may continue to decrease.
For the AMI patients complicated with VSR, should we perform occlusion first or percutaneous coronary intervention (PCI) first?
Expert opinion 1: Dr. Irbaz Hameed and Dr. Arash Salemi
The timing of occlusion and PCI in AMI patients complicated by VSR is at the physicians’ discretion. PCI is generally performed first if the patient presents with unstable angina or if the culprit lesion is visualized in proximal vessels with heavy thrombosis which can predispose to recurrent MI (19). Early PCI can also rescue ischemic myocardium and prevent extension of infarct (20).
However, PCI can be problematic and cause reperfusion injury and further damage the myocardium following infarction, particularly in situations of complete coronary vessel occlusion and absent collateral flow (9,21).
Expert opinion 2: Dr. Gianfranco Butera
Usually it is advisable to perform revascularization before trying to attempt VSD closure or at least as a concomitant procedure. In fact, there is abundant evidence in the literature showing that concomitant revascularization by coronary artery bypass grafting (CABG) to all the stenotic arteries supplying the non-infarcted area improves 30-day mortality (22,23).
In the current era, when possible, it is advisable to perform percutaneous coronary arterial revascularization before attempting closure of the ventricular septal defect (19).
Expert opinion 3: Dr. Evan J. Zucker
PCI is likely to be more time-sensitive in the setting of AMI and thus would generally be expected to be performed first. If the involved coronary territory was small, unlikely to be salvageable, or required a very challenging intervention, while the VSR was more life-threatening and technically approachable, VSR occlusion first might be reasonable. The individual patient circumstances are paramount to the decision.
Why is cardiac MRI more accurate when measuring the size of VSR? Should the cardiac MRI results be used as a basis for selecting an occluder for VSR patients in surgery?
Expert opinion 1: Dr. Irbaz Hameed and Dr. Arash Salemi
Cardiac MRI has previously been utilized for imaging VSDs (24,25). The safety of MRI in patients with previous coronary bare-metal or drug-eluting stents has also been shown (12,13). MRI is a 3D technique that is an improvement over the inherent weaknesses of 2D imaging, as 2D imaging only captures a minimum portion of a particular structure of the heart at a point in time. Consequently, the size of any anatomical defect, such as a VSR, or structure can be underestimated (26,27). MRI can visualize a larger section of tissue around the area of interest. The 3D images can be analyzed from multiple angles and allow calculations of both the area and circumference of VSR on both sides of the septum. 3D transesophageal echocardiography is also a suitable alternative in patients with contraindications to MRI (27).
These measurements have direct implications regarding the type and size of the occluder used for percutaneous or surgical closure of VSR. The volume of aneurysm can also be quantified and acts as a superior parameter in terms of sizing compared to dimensions alone (27,28).
However, cardiac MRI may not be applicable to all patients, and its role in acute post infarction ventricular septal defect is limited. Such patients are severely ill and at risk of heart rate and circulatory instability.
Expert opinion 2: Dr. Gianfranco Butera
Post-myocardial infarction is not a hole with a defined size and rims but an undefined rupture within the interventricular septum. This is why a detailed in vivo description of the dynamic anatomy is relevant in such cases. In fact, there may be extensive variability in the defect characteristics of VSD with thin and akinetic edges while other defects can get larger and some smaller in systole. Only MRI may give information about tissue characteristics, size, and variability during the cardiac cycle, along with insight into the relationship to valves and ventricular walls.
Nowadays, a multimodal imaging approach is needed to plan and perform successful post-myocardial infarction VSD closure (29,30).
Expert opinion 3: Dr. Evan J. Zucker
Cardiac MRI would be expected to be more accurate for VSR sizing compared to echocardiography, facilitating precise preoperative assessment. MRI scans can be prescribed in any plane with excellent contrast resolution. Moreover, MRI can often obviate typical drawbacks of echocardiography such as potentially limited acoustic windows due to body habitus or prior surgery/implanted devices and inter-operator variability. Traditional cardiac MRI techniques can be relatively time-consuming, requiring frequent patient breath-holds and skilled MRI technologists to obtain diagnostic images, with lesser spatial resolution compared to echocardiography. However, new and emerging techniques such as 4D flow MRI allow fast, free-breathing, volumetric acquisitions, requiring limited operator input. These techniques could be particularly useful in the AMI patient who may too unstable or unable to comply with long, breath-held acquisitions.
How to choose a proper occluder when treating VSR?
Expert opinion 1: Dr. Irbaz Hameed and Dr. Arash Salemi
Several factors must be considered in patient and device selection, primarily related to VSR morphology and the relationship to neighboring structures. Specifically designed for post-MI VSR repair, the Amplatzer™ PI Muscular VSD Occluder (St. Jude Medical, St. Paul, MN, USA) is available with a maximum waist size of 24 mm and a disc size of 34 mm (29,31).With the aid of computed tomography and MRI, a 24 mm waist diameter can only occlude 50% of the left side of VSRs, and a 34 mm disc diameter can reach the margins of 75% of the defects in both systole and diastole (29). For percutaneous closure, defects <15 mm are considered optimal but there have been reports of successful repair of larger defects but unsuccessful closure (1,29). The occluding disc may be oversized to improve procedure success by factoring in possible defect enlargement due to tissue necrosis.
Expert opinion 2: Dr. Gianfranco Butera
Currently available devices have a fixed and usually round central waist, which plugs the hole. In the case of post-myocardial infarction VSD, the defect is not round and it is not a hole. Therefore, it may be quite difficult to find rules to follow that would aid in making a decision.
Some help may come from echocardiographic evaluation and MRI study. Furthermore, left ventricular angiography gives more information in order to select the proper device. Finally, in my clinical practice I prefer to balloon size the defect by using the ASD sizing balloon. This is useful to check defect size, to test the defect walls, and rule out the presence of accessory defects. Based upon the diameter of the waist on the sizing balloon, I then add 2 to 4 mm and the device size is subsequently chosen.
Expert opinion 3: Dr. Evan J. Zucker
Many factors should be taken into consideration. The size and geometry of both the VSR and available occluder devices need to be considered. The location of the VSR should be noted, and the device should be able to close the defect with an appropriate landing zone. If repeat MRI may be needed in the future, care should be taken to select a device that is MRI-compatible and would not be expected to create extensive MRI artifacts. The technical proficiency and experience of the interventionalist are also very important; the “second-best” option based on sizing may be the optimal device in practice if the interventionalist is more familiar with it and can more confidently deploy it successfully while minimizing the procedure and fluoroscopy exposure time.
What complications should be particularly paid attention to and prevented during an interventional occlusion?
Expert opinion 1: Dr. Irbaz Hameed and Dr. Arash Salemi
The complications facing occlusion surgery include defects resulting from lack of a circumferential septal rim, defect in characterization, complicated morphology causing serpiginous defects, and early closure following infarction due to tissue instability. Careful assessment of defects and device selection in appropriate patients are essential for successful occlusion of VSR.
Expert opinion 2: Dr. Gianfranco Butera
Several potential complications may occur during VSD transcatheter closure. These complications include arrhythmias, tricuspid leaflet rupture, tamponade subsequent to perforation of the ventricular wall (32), significant residual shunting, device malposition/embolization, and need for immediate open heart surgery.
In order to prevent and avoid potential complications, some special techniques could be used, such as the over the wire device implantation (33) or the goose-neck snare-assisted techniques (34,35).
Expert opinion 3: Dr. Evan J. Zucker
Potential immediate complications, in addition to idiosyncratic sequelae such as arrhythmia and death, include residual shunting or valvular regurgitation related to suboptimal device placement. Additional concerns include partial or complete device breakage, dislocation, or embolization. Vigilance should be maintained for active bleeding or hematoma formation as well as thromboembolic events that may be more likely in the longer term. Post-procedural pericardial effusion development has been reported. In addition, interventionalists should always be cognizant of potential allergies related to intravenously administered iodinated contrast media. Moreover, iatrogenic complications such as dissection, AV fistula, or hematoma formation related to vascular access or manipulation are also potential risks of any interventional procedure.
Conclusions
The effect of interventional therapy for VSR has been widely recognized, and cardiac magnetic resonance can play an important role of the location and size of VSR. There are still, however, some controversies in specific treatment timing and device size selection. Future multicenter studies are required to identify patients best suited for interventional treatment timing. Additionally, further developments in devices and delivery techniques are required in order to optimize interventional outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Jones BM, Kapadia SR, Smedira NG, et al. Ventricular septal rupture complicating acute myocardial infarction: a contemporary review. Eur Heart J 2014;35:2060-8. [Crossref] [PubMed]
- Moreyra AE, Huang MS, Wilson AC, et al. Trends in incidence and mortality rates of ventricular septal rupture during acute myocardial infarction. Am J Cardiol 2010;106:1095-100. [Crossref] [PubMed]
- Noguchi K, Yamaguchi A, Naito K, et al. Short-term and long-term outcomes of postinfarction ventricular septal perforation. Gen Thorac Cardiovasc Surg 2012;60:261-7. [Crossref] [PubMed]
- Shimokawa T. Mechanical complications of acute myocardial infarction. Nihon Rinsho 2011;69:230-4. [PubMed]
- Gassenmaier T, Gorski A, Aleksic I, et al. Impact of cardiac magnet resonance imaging on management of ventricular septal rupture after acute myocardial infarction. World J Cardiol 2013;5:151-3. [Crossref] [PubMed]
- Amin FR, Mandal AK, Al-Obaidi M, et al. Ventricular septal rupture and intraseptal pseudo-aneurysm complicating acute myocardial infarction: management in the multimodality imaging era. Postgrad Med J 2012;88:425-6. [Crossref] [PubMed]
- Dhaliwal S, Ducas R, Shuangbo L, et al. Multimodality cardiac imaging of a ventricular septal rupture postmyocardial infarction: a case report. BMC Res Notes 2012;5:583. [Crossref] [PubMed]
- Menon V, Webb JG, Hillis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. Should we emergently revascularize occluded coronaries in cardiogenic shock? J Am Coll Cardiol 2000;36:1110-6. [Crossref] [PubMed]
- Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I Trial Investigators. Circulation 2000;101:27-32. [Crossref] [PubMed]
- Lemery R, Smith HC, Giuliani ER, et al. Prognosis in rupture of the ventricular septum after acute myocardial infarction and role of early surgical intervention. Am J Cardiol 1992;70:147-51. [Crossref] [PubMed]
- Landzberg MJ, Lock JE. Transcatheter management of ventricular septal rupture after myocardial infarction. Semin Thorac Cardiovasc Surg 1998;10:128-32. [Crossref] [PubMed]
- Sommer T, Maintz D, Schmiedel A, et al. High field MR imaging: magneticfield interactions of aneurysm clips, coronary artery stents and iliac artery stents witha 3.0 Tesla MR system. Rofo 2004;176:731-8. [PubMed]
- Shellock FG, Forder JR. Drug eluting coronary stent: in vitro evaluation of magnet resonance safety at 3 Tesla. J Cardiovasc Magn Reson 2005;7:415-9. [Crossref] [PubMed]
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119-77. [Crossref] [PubMed]
- avies RE, Gilchrist IC. Contemporary Management of Post-MI Ventricular Septal Rupture. [cited 2019 Jun 3]. Available online: https://www.acc.org/latest-in-cardiology/articles/2018/07/30/06/58/contemporary-management-of-post-mi-ventricular-septal-rupture
- Arnaoutakis GJ, Zhao Y, George TJ, et al. Surgical repair of ventricular septal Defect after myocardial infarction: outcomes from the society of thoracic surgeons national database. Ann Thorac Surg 2012;94:436-43. [Crossref] [PubMed]
- Omar S, Morgan GL, Panchal HB, et al. Management of post-myocardial infarction ventricular septal defects: A critical assessment. J Interv Cardiol 2018;31:939-48. [Crossref] [PubMed]
- Zhu XY, Qin YW, Han YL, et al. Long-term efficacy of transcatheter closure of ventricular septal defect in combination with percutaneous coronary intervention in patients with ventricular septal defect complicating acute myocardial infarction: a multicentre study. EuroIntervention 2013;8:1270-6. [Crossref] [PubMed]
- Bueno H, Martínez-Sellés M, Pérez-David E, et al. Effect of thrombolytic therapy on the risk of cardiac rupture and mortality in older patients with first acute myocardial infarction. Eur Heart J 2005;26:1705-11. [Crossref] [PubMed]
- Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473-81. [Crossref] [PubMed]
- Barker TA, Ramnarine IR, Woo EB, et al. Repair of post-infarct ventricular septal defect with or without coronary artery bypass grafting in the northwest of England: a 5-year multi-institutional experience. Eur J Cardiothorac Surg 2003;24:940-6. [Crossref] [PubMed]
- Perrotta S, Lentini S. In patients undergoing surgical repair of post-infarction ventricular septal defect, does concomitant revascularization improve prognosis? Interact Cardiovasc Thorac Surg 2009;9:879-87. [Crossref] [PubMed]
- Thuny F, Jacquier A, Riberi A, et al. Images in cardiovascular medicine. Ventricular septal rupture after a nonpenetrating chest trauma: findings from real-time three-dimensional echocardiography and cardiac magnetic resonance. Circulation 2005;112:e339-40. [Crossref] [PubMed]
- Smíd M, Ferda J, Zlocha V. Charles University Prague Research Project MSM nr. 0021620817 Investigators. Post-traumatic ventricular septal defect. Eur Heart J 2008;29:575. [PubMed]
- Nanda NC, Rahman SMAE, Khatri G, et al. Incremental Value of Three-Dimensional Echocardiography Over Transesophageal Multiplane Two-Dimensional Echocardiography in Qualitative and Quantitative Assessment of Cardiac Masses and Defects. Echocardiography 1995;12:619-28. [Crossref]
- Arisha MJ, Hsiung MC, Nanda NC, et al. Incremental value of live/real time three-dimensional transesophageal echocardiography in the assessment of ventricular septal rupture following acute myocardial infarction. Echocardiography 2017;34:1680-6. [Crossref] [PubMed]
- Gassenmaier T, Gorski A, Aleksic I, et al. Impact of cardiac magnet resonance imaging on management of ventricular septal rupture after acute myocardial infarction. World J Cardiol 2013;5:151-3. [Crossref] [PubMed]
- Hamilton MCK, Rodrigues JCL, Martin RP, et al. The in vivo morphology of post-infarct ventricular septal defect and the implications for closure. JACC Cardiovasc Interv 2017;10:1233-43. [Crossref] [PubMed]
- Iyer S, Bauer T, Yeung M, et al. A heart team and multi-modality imaging approach to percutaneous closure of a post-myocardial infarction ventricular septal defect. Cardiovasc Diagn Ther 2016;6:180-4. [Crossref] [PubMed]
- Schlotter F, de Waha S, Eitel I, et al. Interventional post-myocardial infarction ventricular septal defect closure: a systematic review of current evidence. EuroIntervention 2016;12:94-102. [Crossref] [PubMed]
- Sabiniewicz R, Huczek Z, Zbroński K, et al. Percutaneous Closure of Post-Infarction Ventricular Septal Defects-An Over Decade-long Experience. J Interv Cardiol 2017;30:63-71. [Crossref] [PubMed]
- Butera G, Castaldi B, McDonald ST. Over the wire technique device implantation. Catheter Cardiovasc Interv 2012;80:485-92. [Crossref] [PubMed]
- Butera G, Lovin N, Basile DP, et al. Goose-neck snare-assisted transcatheter ASD closure: A safety procedure for large and complex ASDs. Catheter Cardiovasc Interv 2016;87:926-30. [Crossref] [PubMed]
- Faccini A, Butera G. Techniques, Timing, and Prognosis of Transcatheter Post myocardial Infarction Ventricular Septal Defect Repair. Curr Cardiol Rep 2019;21:59. [Crossref] [PubMed]