
Differential diagnosis between lymphoma-associated malignant pleural effusion and tuberculous pleural effusion
Introduction
Most malignant pleural effusions (MPE) occur secondary to pleural metastasis of lung cancer, breast cancer, and lymphomas (1,2). Pleural fluid cytology is considered the initial step in the diagnosis of MPE. However, the diagnostic yield for pleural fluid cytology provides a sensitivity of 50–70% and is affected by tumor type and tumor burden, etc. (3). Notably, lymphoma-associated MPE (L-MPE) has a relatively lower cytological yield compared with other solid tumors such as adenocarcinoma of lung (1,4,5). Thus, the cause of effusion is more likely to remain undiagnosed after initial pleural fluid analysis in these patients (5). These patients represent a true challenge in the differential diagnosis of lymphocytic pleural effusions.
Pleural fluid adenosine deaminase (ADA) is a well-known biomarker for the diagnosis of tuberculous pleural effusion (TPE) in patients with lymphocytic exudative pleural effusion (6-8). However, elevated pleural fluid ADA levels, a finding that is uncommon in solid tumors, is frequently found in patients with L-MPE (8,9). This finding can further complicate the differential diagnosis between L-MPE and TPE, especially in areas with a high prevalence of tuberculosis (TB). Thus, some lymphoma patients may be misdiagnosed with TPE and are unnecessarily treated with potentially harmful anti-TB medication (10,11). Therefore, the presence of certain discriminating factors between patients with L-MPE and TPE would help in guiding the selection of patients who require further evaluation including pleural biopsy.
The objective of this study was to compare clinical, laboratory, and pleural fluid characteristics to identify useful variables for the differential diagnosis of L-MPE and TPE.
Methods
Study population and design
This retrospective study was conducted using the Pleural Diseases database of the Kyungpook National University Hospital, a tertiary referral hospital in South Korea in an area with an intermediate prevalence of active TB cases (12). All consecutive patients who were diagnosed with L-MPE or TPE between January 2011 and December 2016 were included. First, the following criteria were used to diagnose L-MPE in patients with pathologically confirmed malignant lymphoma: (I) identification of malignant cells in pleural fluid or tissue; or (II) pleural nodularity clearly detected by computed tomography (CT) with increased pleural F-18 fluorodeoxyglucose uptake revealed by positron emission tomography (PET) scan, response to chemotherapy, and no evidence for other causes of exudative pleural effusion (13,14). Second, a diagnosis of TPE was made when one of the following criteria was met: (I) positive culture for Mycobacterium tuberculosis in pleural fluid, pleural tissue, sputum, or bronchial aspirate; or (II) pathological finding of chronic granulomatous pleural inflammation without evidence of other granulomatous disease (6).
Details regarding patient demographics, clinical symptoms, and laboratory and pleural fluid data were obtained from electronic medical records. Routine tests for pleural effusion in the study hospital included total leukocyte cell with differential cell count, biochemical studies of lactate dehydrogenase (LDH), protein, and glucose, microbiological studies (Gram stain, aerobic/anaerobic culture, fungus culture, acid-fast stain, and mycobacterial culture), ADA, pH, and cytology. LDH levels were determined by the LDH test reagent [lactate dehydrogenase acc. to IFCC ver.2 (LDHI2), Roche Diagnostics, Mannheim, Germany] using a COBAS 8000 analyzer (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Pleural fluid ADA activity was measured in a routine clinical setting using an automated calorimetric assay kit (Runpia Liquid ADA, Kyokuto Pharmaceutical Industrial Co., Ltd., Japan) as described in the package insert.
The study protocols were reviewed and approved by the Institutional Review Board of Kyungpook National University Hospital.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as the median (interquartile range) and were analyzed using the Mann-Whitney test. Categorical variables were expressed as absolute values and percentages, and were analyzed using the Chi square or Fisher’s exact tests. To identify independent predictive variables for L-MPE, variables with P<0.05 on univariate analysis were entered into a multivariate conditional logistic regression model. The goodness of fit of the model was assessed by means of the Hosmer and Lemeshow test. Data for continuous variables were converted to categorical variables based on optimal cut-off values using the receiver operating characteristic (ROC) curve analysis. Diagnostic accuracy was assessed from the area under the curve (AUC). A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Thirty lymphoma patients who underwent diagnostic thoracentesis due to pleural effusion between January 2011 and December 2016 were reviewed. After excluding 13 lymphoma patients with transudative pleural effusion (n=4), parapneumonic effusion (n=3), TPE (n=2), drug (tacrolimus)-induced effusion (n=1), and uncertain causes (n=3), 17 patients were eligible for the L-MPE group. Malignant cells were identified in pleural fluid cytology and/or pleural tissue of four patients. The remaining 13 patients with pathologically confirmed malignant lymphoma were diagnosed with L-MPE based on compatible evidence of pleural involvement of lymphoma on chest CT and PET scan, response to chemotherapy, and exclusion of other etiologies for pleural effusion. In terms of pathology types in the L-MPE group, 13 patients had B-cell non-Hodgkin lymphoma (NHL) and 4 had T-cell NHL.
The TPE group included 216 patients with a definite diagnosis of TPE during the study period; positive microbiological [n=186: pleural fluid (n=67), sputum (n=84), and bronchial aspirate (n=35)] and histologic results (n=30).
Comparison of clinical, laboratory, and pleural fluid data between two groups
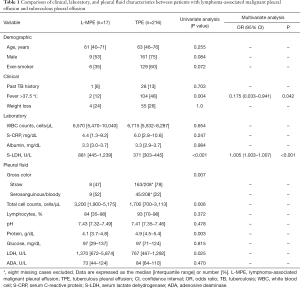
The clinical, laboratory, and pleural fluid characteristics of the study population are shown in Table 1. Median age was 61 and 63 years in the L-MPE and TPE groups, respectively. Male and ever-smokers tended to be more frequent in patients with TPE. Fever, which was defined as an axillary temperature >37.5 °C, was significantly more frequent in patients with TPE compared with those with L-MPE (48% vs. 12%, P=0.004). There was no difference in frequency of body weight loss (more than 10% over past 6 months) in both groups.
Full table
In terms of laboratory findings, white blood cell count and serum C-reactive protein (CRP) and albumin levels were similar between the two groups. However, serum LDH levels were significantly higher in patients with L-MPE than those with TPE (881 vs. 371 U/L, P<0.001).
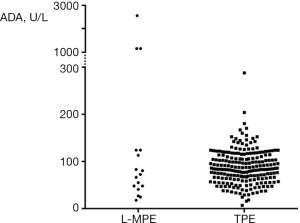
With regard to the gross appearance of pleural fluid, blood-stained (serosanguinous or bloody) effusions were more frequent in patients with L-MPE compared with those with TPE (52% vs. 22%, P=0.007). There was no case with chylous or pseudochylous effusion. Total leukocyte count of pleural fluid was greater in patients with L-MPE than those with TPE (3,200 vs. 1,700 cells/µL, P=0.006). Pleural fluid protein levels were more elevated in TPE than L-MPE (4.9 vs. 4.1 g/dL, P=0.003), while pleural fluid LDH levels were greater in L-MPE than TPE (1,370 vs. 767 U/L, P=0.025). There was no difference in the proportion of lymphocytes and levels of pH and glucose of pleural fluid. Median ADA levels in pleural fluid were 73 and 84 U/L in patients with L-MPE and TPE, respectively. Approximately 80% of L-MPE patients (14/17) had ADA levels exceeding the usual cut-off values (40 U/L) for TPE. ADA levels widely varied in patients with L-MPE (Figure 1). In particular, patients with T-cell histology type showed markedly higher ADA levels compared with those with B-cell histology type (1,177 vs. 55 U/L, P=0.003). There was no significant difference in serum and pleural fluid parameters except pleural fluid pH and glucose levels between patients with TPE with a positive mycobacterial culture on any respiratory specimens (sputum or bronchial aspirate) and without (data not shown).
In the multivariate analysis, fever was negatively associated with L-MPE [odds ratio (OR): 0.175, 95% confidence interval (CI): 0.033–0.941, P=0.042], while serum LDH levels were positively associated with L-MPE (OR: 1.005, 95% CI: 1.003–1.007, P<0.001).
Diagnostic performance of independent variables for differentiation of two groups
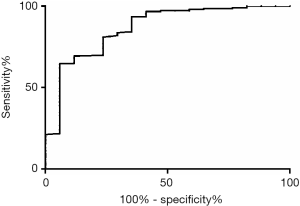
Diagnostic performance of two independent variables obtained from the multivariate analysis is presented in Table 2. The optimal cut-off value of serum LDH levels to differentiate L-MPE and TPE was assessed using ROC curve analysis (Figure 2). At a cut-off value of 460 U/L, serum LDH had a sensitivity of 76% and a specificity of 81% with AUC 0.79 (0.67–0.91). Serum LDH levels >460 U/L without fever provided a sensitivity of 65%, a specificity of 89%, a positive predictive value of 31%, and a negative predictive value (NPV) of 97%. There was no case with both fever and serum LDH ≤460 U/L in patients with L-MPE, providing an NPV of 88%.

Full table
Discussion
The main findings of the current study are as follows. First, overall, patients with L-MPE and TPE presented very similar clinical, laboratory, and pleural fluid characteristics. Second, pleural fluid ADA levels were frequently elevated in both groups, and were not significantly different between the two groups. However, markedly higher ADA levels suggested L-MPE due to T-cell NHL rather than TPE. Third, in the multivariate analysis, fever was negatively associated with L-MPE, while serum LDH levels were positively associated with L-MPE. Finally, serum LDH >460 U/L provided a sensitivity of 76% and a specificity of 81% to distinguish L-MPE and TPE. A combination of no fever and serum LDH >460 U/L increased the specificity to 89%.
This study shows that patients with L-MPE and TPE require careful evaluation for differentiation. Actually, three (18%) of 17 patients with L-MPE in this study were considered to have TPE before the histological confirmation of lymphoma. Many probable TPE cases are administered empirical anti-TB treatment based on lymphocytic exudate with high pleural fluid ADA levels, but without other definite evidence of TB in TB-prevalent areas; however, our findings indicate that some of these patients may potentially have L-MPE and differential diagnosis in these patients is important. Fever and serum LDH levels may help physicians make clinical decisions for investigating other additional findings in this challenging situation, although they had modest diagnostic performance.
Most patients with NHL present with painless lymphadenopathy with or without non-specific constitutional symptoms such as weight loss, fever, and night sweats (15). These so-called B-symptoms are reported in approximately 20–30% of patients with NHL (16,17). Interestingly, these systemic symptoms are also common manifestations of TB (18). Thus, constitutional symptoms between lymphoma and TB may not be particularly different. In this study, any one of B-symptoms were noted in over 30% of patients with L-MPE, consistent with the frequency reported by previous studies (16,17). However, our study showed that fever was four times more common in patients with TPE than in those with L-MPE. This suggests that the presence of fever is more likely to favor TPE over L-MPE in the setting of lymphocytic exudate with elevated pleural fluid ADA levels.
Lymphoma is one of several representative diseases presenting elevated ADA levels among non-TB effusions (8,9). Consequently, this study showed that pleural fluid ADA levels were similarly elevated in patients with L-MPE and TPE. Patients with underlying lymphoma have higher risk of TB than those with other solid malignancy (19). Thus, in patients with lymphocytic pleural effusion during the course of the lymphoma, it should be kept in mind that elevated pleural fluid ADA levels can be caused by concurrent TPE as well as lymphoma progression. Nevertheless, it is noteworthy that markedly higher pleural fluid ADA levels (>1,000 U/L) was found to be associated with L-MPE of T-cell type, rather than TPE. This finding is plausible, given that T-lymphocytes have much greater ADA activity than B-lymphocytes (20) and high serum ADA levels are a feature of immature T-cell lymphoma and T-cell leukemia (21).
Serum CRP, a biomarker of the systemic inflammatory response, has been shown to be useful to differentiate TPE from MPE (22,23). Serum CRP levels are usually lower in MPE than TPE. However, there was no significant difference in serum CRP levels between L-MPE and TPE patients in this study. When considering that serum CRP levels have been found to be a prognostic marker in NHL (24,25), L-MPE patients having widespread disease are likely to have elevated serum CRP levels. Thus, serum CRP levels failed to discriminate between L-MPE and TPE.
LDH, an enzyme converting glucose from food into usable energy for cells, is commonly elevated in patients with lymphoma as well as sepsis and other malignancies (26-28). The rise of serum LDH in cancer is explained by the preferential use of glycolysis for energy, which is mediated by LDH, because glycolysis is advantageous to growing tumor cells compared to oxidative phosphorylation (29,30). Thus, serum LDH levels reflect tumor burden and cellular turnover in NHL and have prognostic value. Patients with lymphoma with accompanying MPE generally have widespread disease (5). Given this advanced lymphoma status, higher serum LDH levels are not surprising in the L-MPE group. Elevated serum LDH levels may be meaningful as a significant predictor for L-MPE in situations where serum CRP and pleural fluid ADA levels no longer play a role in differential diagnosis between particular type of MPE, i.e., L-MPE and TPE studied herein. Thus, with a good specificity (89%), elevated serum LDH levels without fever may play a role in distinguishing between L-MPE and TPE. This finding is supported by a recent report showing that serum LDH was raised to significantly higher levels in patients with MPE, discriminating between MPE and TPE (31).
The gross appearance of pleural fluid may provide initial diagnostic clues (32). Chylothorax can be caused by obstruction of the thoracic duct due to lymphoma (33), but there was no case with chylous effusion in this study. Also, multivariate analysis failed to show the association between the gross appearance and cause of effusions although blood-stained effusions were more frequently found in patients with L-MPE than in those with TPE.
Our study has limitations that should be considered. First, it is a retrospective study, with inevitable selection bias. Second, the small number of cases with L-MPE reflects the diagnostic difficulty in these patients. Pleural effusion in patients with lymphoma can occur due to causes other than pleural involvement of the lymphoma, such as obstruction of lymphatic return by enlarged lymph nodes. Only some of the patients with L-MPE in this study were cytohistologically confirmed. However, the remaining patients had pleural effusion due to pleural infiltration by the lymphoma as seen on CT and PET scan. Pleural involvement of lymphoma can be detected by using flow cytometry of pleural fluid (34) although it is not routinely performed in our institution. Adoption of flow cytometric immunophenotyping method would provide a helpful information to distinguish between L-MPE and TPE. Third, the current study did not measure isoenzymes of ADA, ADA1 and ADA2, which may differ between L-MPE and TPE (8,35) and may be helpful in differentiating between these two diseases. However, ADA isoenzymes are not measured in routine clinical practice. Lastly, the present study did not include other diseases that may increase serum and pleural fluid LDH levels, such as parapneumonic effusion or MPE from other primary sites (31,36,37). Thus, our results could not be extrapolated to all cases of pleural effusion, although variations in the elevation of serum LDH levels may be helpful in these differential diagnoses.
Conclusions
Patients with L-MPE and TPE present very similar clinical, laboratory, and pleural fluid characteristics. Serum CRP and pleural fluid ADA levels, commonly used for discrimination between MPE and TPE, did not distinguish L-MPE and TPE. In contrast, despite limited diagnostic performance, fever and serum LDH levels may be helpful in guiding the differential diagnosis of L-MPE and TPE. Lymphoma should be kept in mind in the differential diagnosis if there is no fever but elevated serum LDH levels >460 U/L, in patients with lymphocytic pleural effusion and high ADA levels.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocols were reviewed and approved by the Institutional Review Board of Kyungpook National University Hospital and written informed consent was waived by the ethics committee.
References
- American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987-2001. [Crossref] [PubMed]
- Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985;56:905-9. [Crossref] [PubMed]
- Porcel JM, Light RW. Pleural effusions. Dis Mon 2013;59:29-57. [Crossref] [PubMed]
- Das DK. Serous effusions in malignant lymphomas: a review. Diagn Cytopathol 2006;34:335-47. [Crossref] [PubMed]
- Alexandrakis MG, Passam FH, Kyriakou DS, et al. Pleural effusions in hematologic malignancies. Chest 2004;125:1546-55. [Crossref] [PubMed]
- Light RW. Update on tuberculous pleural effusion. Respirology 2010;15:451-8. [Crossref] [PubMed]
- Liang QL, Shi HZ, Wang K, et al. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med 2008;102:744-54. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Bielsa S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med 2010;21:419-23. [Crossref] [PubMed]
- Antonangelo L, Vargas FS, Genofre EH, et al. Differentiating between tuberculosis-related and lymphoma-related lymphocytic pleural effusions by measuring clinical and laboratory variables: is it possible? J Bras Pneumol 2012;38:181-7. [Crossref] [PubMed]
- Puvaneswaran B, Shoba B. Misdiagnosis of tuberculosis in patients with lymphoma. S Afr Med J 2012;103:32-3. [Crossref] [PubMed]
- Masamba LPL, Jere Y, Brown ERS, et al. Tuberculosis Diagnosis Delaying Treatment of Cancer: Experience From a New Oncology Unit in Blantyre, Malawi. J Glob Oncol 2016;2:26-9. [Crossref] [PubMed]
- World Health Organization. Tuberculosis. Available online: http://www.who.int/tb/country/data/profiles/en/
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis. Acad Radiol 2014;21:11-20. [Crossref] [PubMed]
- Yao CW, Wu BR, Huang KY, et al. Adenosine deaminase activity in pleural effusions of lymphoma patients. QJM 2014;107:887-93. [Crossref] [PubMed]
- Armitage JO, Gascoyne RD, Lunning MA, et al. Non-Hodgkin lymphoma. Lancet 2017;390:298-310. [Crossref] [PubMed]
- Aoki T, Izutsu K, Suzuki R, et al. Prognostic significance of pleural or pericardial effusion and the implication of optimal treatment in primary mediastinal large B-cell lymphoma: a multicenter retrospective study in Japan. Haematologica 2014;99:1817-25. [Crossref] [PubMed]
- Chen YP, Huang HY, Lin KP, et al. Malignant Effusions Correlate With Poorer Prognosis in Patients With Diffuse Large B-Cell Lymphoma. Am J Clin Pathol 2015;143:707-15. [Crossref] [PubMed]
- Dheda K, Barry CE, Maartens G. Tuberculosis. Lancet 2016;387:1211-26. [Crossref] [PubMed]
- Kamboj M, Sepkowitz KA. The risk of tuberculosis in patients with cancer. Clin Infect Dis 2006;42:1592-5. [Crossref] [PubMed]
- Huang AT, Logue GL, Engelbrecht GL. Two biochemical markers in lymphocyte subpopulations. Br J Haematol 1976;34:631-8. [Crossref] [PubMed]
- Ganeshaguru K, Lee N, Llewellin P, et al. Adenosine deaminase concentrations in leukaemia and lymphoma: relation to cell phenotypes. Leuk Res 1981;5:215-22. [Crossref] [PubMed]
- Lee J, Lee YD, Lim JK, et al. Predictive Factors and Treatment Outcomes of Tuberculous Pleural Effusion in Patients With Cancer and Pleural Effusion. Am J Med Sci 2017;354:125-30. [Crossref] [PubMed]
- Chierakul N, Kanitsap A, Chaiprasert A, et al. A simple C-reactive protein measurement for the differentiation between tuberculous and malignant pleural effusion. Respirology 2004;9:66-9. [Crossref] [PubMed]
- Legouffe E, Rodriguez C, Picot MC, et al. C-reactive protein serum level is a valuable and simple prognostic marker in non Hodgkin's lymphoma. Leuk Lymphoma 1998;31:351-7. [Crossref] [PubMed]
- Troppan KT, Schlick K, Deutsch A, et al. C-reactive protein level is a prognostic indicator for survival and improves the predictive ability of the R-IPI score in diffuse large B-cell lymphoma patients. Br J Cancer 2014;111:55-60. [Crossref] [PubMed]
- Fasola G, Fanin R, Gherlinzoni F, et al. Serum LDH concentration in non-Hodgkin's lymphomas. Relationship to histologic type, tumor mass, and presentation features. Acta Haematol 1984;72:231-8. [Crossref] [PubMed]
- Hong J, Yoon HH, Ahn HK, et al. Prognostic role of serum lactate dehydrogenase beyond initial diagnosis: a retrospective analysis of patients with diffuse large B cell lymphoma. Acta Haematol 2013;130:305-11. [Crossref] [PubMed]
- Goldman RD, Kaplan NO, Hall TC. Lactic Dehydrogenase in Human Neoplastic Tissues. Cancer Res 1964;24:389-99. [PubMed]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004;4:891-9. [Crossref] [PubMed]
- Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001;292:504-7. [Crossref] [PubMed]
- Verma A, Abisheganaden J, Light RW. Identifying Malignant Pleural Effusion by A Cancer Ratio (Serum LDH: Pleural Fluid ADA Ratio). Lung 2016;194:147-53. [Crossref] [PubMed]
- Villena V, Lopez-Encuentra A, Garcia-Lujan R, et al. Clinical implications of appearance of pleural fluid at thoracentesis. Chest 2004;125:156-9. [Crossref] [PubMed]
- Doerr CH, Allen MS, Nichols FC 3rd, et al. Etiology of chylothorax in 203 patients. Mayo Clin Proc 2005;80:867-70. [Crossref] [PubMed]
- Bode-Lesniewska B. Flow Cytometry and Effusions in Lymphoproliferative Processes and Other Hematologic Neoplasias. Acta Cytol 2016;60:354-64. [Crossref] [PubMed]
- Bae MJ, Ryu S, Kim HJ, et al. Mycobacterium tuberculosis ESAT6 and CPF10 Induce Adenosine Deaminase 2 mRNA Expression in Monocyte-Derived Macrophages. Tuberc Respir Dis (Seoul) 2017;80:77-82. [Crossref] [PubMed]
- Lee J, Yoo SS, Lee SY, et al. Pleural fluid adenosine deaminase/serum C-reactive protein ratio for the differentiation of tuberculous and parapneumonic effusions with neutrophilic predominance and high adenosine deaminase levels. Infection 2017;45:59-65. [Crossref] [PubMed]
- Kim CH, Park JE, Yoo SS, et al. Usefulness of serum lactate dehydrogenase/pleural fluid adenosine deaminase ratio for differentiating Mycoplasma pneumoniae parapneumonic effusion and tuberculous pleural effusion. J Infect 2017;75:581-3. [Crossref] [PubMed]


