
High Aβ load may cause microglial cell dysfunction and reduced nuclear repressor element-1 silencing transcription factor (REST) expression which might be ascribed to its degradation by ubiquitination
Introduction
Alzheimer’s disease (AD) is a major cause of cognitive disorder in the elderly. Aβ is a pathogenic substance for AD and excess production and abnormal deposition of Aβ have been found as a major mechanism underlying the pathogenesis of AD. However, the time from the Aβ induced pathology to the presence of clinical symptoms is very long and there are a lot of triggering factors involved in this process. Recent years, studies indicate immune dysfunction may be also involved in the pathogenesis of AD (1-3). In 2017, a genome-wide association study on AD showed three new genetic variants and these genes encode three proteins in the microglial cells (4). Microglial cells are phagocytes in the brain responsible for the removal of injured neurons and harmful substances (such as toxic proteins). However, a recent study indicates dysfunctional microglial cells may present excessive engulfment of synapses during the removal of Aβ. In addition, astrocytes and cerebral vascular endothelial cells may aid the microglial cells to exert effects on the cerebral inflammation, causing chronic stress and the subsequent presence and development of clinical symptoms of AD.
Repressor element-1 silencing transcription factor (REST) is involved in the regulation of response to neuroprotective stress, and this response is crucial for the maintenance of cognition during aging. Under stress, the expression of REST in the nucleus of neurons is up-regulated, which may increase the expression of FOXO (a transcription factor) to regulate the response to oxidative stress. As an important transcriptional repressor, REST may bind to the double-helical DNA of RE1 with conservative sequence, which may negatively regulate the expression of neuron-specific genes. Besides the regulation of oxidative stress, REST can also inhibit the expression of genes related to the pathology of AD and cell death, which protect neurons from Aβ induced neurotoxicity (5). However, in case of AD, the REST expression is almost deficient in neurons. Thus, we speculate that specific chronic inflammation may induce the biochemical alterations related to the AD, in which neurons and microglial cells present new biological features, causing neurodegeneration and the presence of dementia.
In the present study, microglial cells and neurons were independently treated with Aβ at different concentrations, and the microglial activation and neuronal toxicity were assessed by morphological observation. In addition, the REST expression was detected in neurons, aiming to explore the mechanism underlying the initiation of AD.
Methods
Ethics declaration
All the procedures were done according to the Guideline for Experimental Animal Use and Care as well as the Declaration of Helsinki.
Cell culture and study design
Microglial cell viability, autophagy and apoptosis examinations
Aβ protein was dissolved in water containing 0.4% DMSO and the resultant solution was named 10-3M storage solution and incubated for 3–4 d at 37 °C for oligomerization. Then, aliquot solution was stored at −80 °C. One hundred µL of BV2 cells or N2A cells were seeded into 96-well plates at a density of 1×104 cells/mL and maintained in DMEM containing 10% FBS for 12 h. Then, Aβ storage solution was added at a final concentration of 1, 2.5 or 5 µM. Cells were further grown for 24 and 48 h, followed by addition of 10 µL of CCK-8 solution. After incubation for 30 min, the absorbance was detected at 450 nm in a microplate reader. Cells were subsequently seeded into flasks at a density of 1×104 cells/mL and Aβ storage solution was added at a final concentration of 1, 2.5 and 5 µM. Twenty-four h later, cells were observed under a light microscope. Then, cells were stained with 1 µg/mL acridine orange (AO) for 20 min, and the autophagic cells were observed under a fluorescence microscope. After staining with 1 µg/mL Hoechst 33258 for 10 min, apoptotic cells were observed under a fluorescence microscope. In addition, cells were harvested, and total proteins were extracted after cell lysis. Then, proteins were subjected to SDS-PAGE and then transferred onto PVDF membranes. The membrane was blocked in non-fat milk for 1 h and incubated with primary antibody overnight. After washing in PBST thrice, the membrane was incubated with secondary antibody for 1 h. After washing in PBST thrice, visualization was done by enhanced chemiluminescence (ECL) and protein bands were scanned for further analysis.
Neuronal viability, autophagy and apoptosis examinations
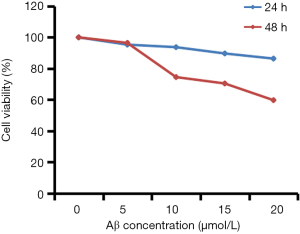
After treatment with Aβ at different concentrations for 48 h, cells in logarithmic growth phase were collected after digestion and re-suspended at [1–2] ×105/mL. The sing cell suspension was added to 96-well plates (100 µL/well) and there were 5 wells in each group. Cells were incubated at 37 °C in an environment with 5% CO2. When cell confluence reached near 100%, cells were treated with Aβ at different concentrations for 48 h. After addition of CCK-8 solution, cells were incubated in dark for 2 h. The optical density was measured at 450 nm in a microplate reader for the assessment of cell viability. In addition, cells were stained with 1 µg/mL AO for 20 min and then autophagic cells were observed under a fluorescence microscope. After staining with 1 µg/mL Hoechst 33258 for 10 min, the apoptotic cells were observed under a fluorescence microscope.
Detection of REST and casein kinase 1 (CK1) expression
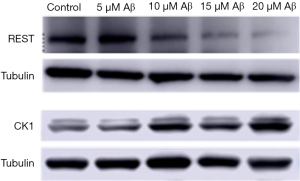
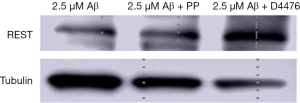
N2A cells were treated with Aβ at different concentrations for 48 h and then incubated with RIPA lysis buffer for 30 min on ice. After centrifugation at 4 °C, the supernatant was collected. The protein concentration was determined with bicinchoninic acid assay (BCA) method. Then, 30 µg of proteins was subjected to separation by SDS-PAGE and then transferred onto PVDF membrane which was blocked in 5% non-fat milk for 2 h. Subsequently, the membrane was incubated with primary antibody at 4 °C overnight and then with secondary antibody. The protein bands were visualized and scanned for the assessment of REST and CK1 expression. After treatment with 2.5 µM Aβ for 48 h, cells were incubated with CK1 agonist [pyrvinium pamoate (PP)] or CK1 inhibitor (D4476) for 2 h. The cell viability was determined with CCK-8 assay. Total protein was extracted from these cells for further detection of REST and CK1 expression by Western blotting.
Animals and study design
Grouping
Six-month-old APP/PS1 transgenic mice with spontaneous AD were subjected to neurological scoring, Morris water maze test and brain pathological examination for the assessment of Aβ deposition in the brain. Neurological scoring was done by swimming, wire hanging and rod walking examinations, and results showed absence of cognitive impairment. The eligible mice were included in model group and wild type mice of the same litter in control group. Transgenic mice were purchased from Nanjing Model Animal Center, housed in specific pathogen free environment with 12 h/12 h light/dark cycle and given ad libitum access to water and food.
Detection of apoptotic cells by TUNEL staining
Sagittal sections with hippocampus, frontal lobe and parietal lobe were selected for the detection of apoptotic cells by TUNEL staining (Wuhan Boster Biotech Co., Ltd). Two sections were used from each animal, and 4 randomly selected sections were examined at a high magnification, and a total of 100 neurons were counted in each section. The proportion of apoptotic neurons was calculated as the apoptosis rate (NAI): NAI = apoptotic neurons (N)/total cells (T) ×100%.
Immunofluorescence staining for REST and CK1
Eight-µm sections were prepared and incubated at room temperature for 30 min. After washing in 1× PBS thrice (3 min for each), sections were blocked in 2% bovine serum albumin (BSA) for 1 h at room temperature. After incubation with REST antibody at 1:100 overnight at 4 °C, sections were washed in 1× PBS thrice (3 min for each) and then incubated with FITC-conjugated goat anti-rabbit secondary antibody at 1:500 (IgG H&L) for 1 h at room temperature. These sections were subjected to staining with DAPI for 3 min. Following washing in 1× PBS thrice (3 min for each), sections were mounted with glycerin, then observed under a fluorescence microscope and photographed. Two sections were used from each animal, and 3 randomly selected fields were examined at a high magnification (400×). The REST and CK1 expression were then localized in cells.
Real-time fluorescence quantitative PCR and Western blotting were performed for the detection of mRNA and protein expression of target genes, respectively. Confocal microscopy immunofluorescence assay was employed to localize the REST expression.
Statistical analysis
Statistical analysis was performed with SPSS version 22.0 and quantitative data are expressed as mean ± standard deviation (SD). Latency in Morris water test was compared with t-test, and a value of P<0.05 was considered statistically significant. Apoptosis rate was compared with Chi-square test and a value of P<0.05 was considered statistically significant.
Results
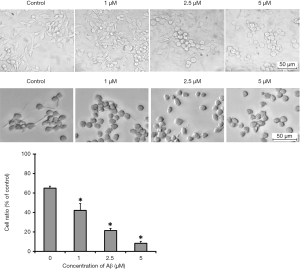
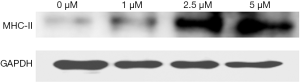
The cell morphology changed significantly, and the proportion of amoebic microglia and the protein expression of major histocompatibility complex class II (MHC-II, a marker of microglial activation) increased with the increase in Aβ concentration (Figures 1,2).
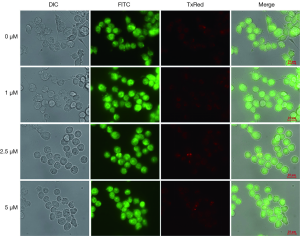
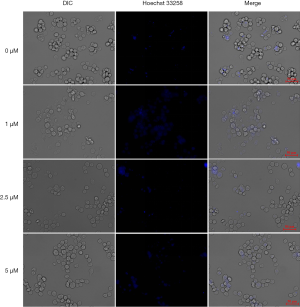
Aβ at different concentrations fails to induce autophagy and apoptosis of microglia. (Figures 3,4).
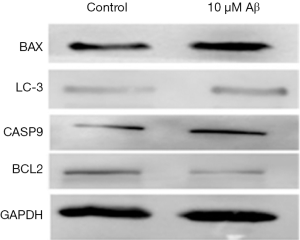
After treatment with Aβ at different concentrations, the N2A cell viability reduced gradually, the expression of BAX, CASP9 and LC-3 increased, but the BCL2 expression reduced (Figures 5,6).
After treatment with Aβ at different concentrations, the REST expression reduced, CK1 expression increased, and both changed in Aβ concentration dependent manner (Figure 7).
After CK1 agonist (PP) treatment, REST expression remained unchanged, but CK1 inhibitor (D4476) treatment significantly increased REST expression (Figure 8).
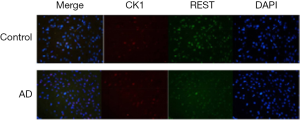
In the neurons of APP/PS1 transgenic mice aged 6 months, the nuclear REST expression reduced but CK1 expression increased, and there was co-expression of CK1 and REST (Figure 9).
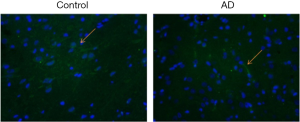
The nuclear REST expression reduced, but the cytoplasmic REST expression remained unchanged in the neurons of 6-month old APP/PS1 transgenic mice (Figure 10).
Discussion
Results of this study showed: (I) Aβ at a concentration low (2.5 µmol/L) could alter the microglial morphology, and the proportion of amoebic microglia and protein expression of MHC-II increased in an Aβ concentration dependent manner; (II) Aβ at a high concentration (10 µmol/L) was able to reduce REST expression, elevate the expression of pro-apoptotic and pro-autophagic genes, inhibit the expression of anti-apoptotic gene, and reduce neuronal activity; (III) REST expression reduced, but CK1 (a phosphorylase specific to REST) increased in neurons, and CK1 inhibitor significantly increased REST expression; (IV) there was co-expression of REST and CK1 in the brain of AD mice aged 6 months, which was characterized by reduced nuclear REST expression and elevated CK1 expression.
Studies have comprehensively investigated the chronic inflammation and revealed some potential targets for the treatment (6-8). The mechanism by which the extensive specific chronic inflammation induce specific symptoms of AD is still unclear and also a challenge in future studies. In the present study, microglial cells were treated with Aβ at different concentrations. Our results showed Aβ at a low concentration could activate microglial cells which then phagocytize Aβ, exerting neuroprotective effects. Under this condition, the neuronal activity remained unchanged. The number of activated microglial cells increased with the elevation of Aβ concentration. The excess activation of microglial cells may induce inflammation and release a large amount of pro-inflammatory cytokines, causing chronic stress to the neurons. Our results showed the neuronal activity reduced significantly after treatment with Aβ at 10 µmol/L. There is evidence showing that toxic proteins such as Aβ may bind to the surface receptors Trem2 and Tyrobp on the microglial cells to activate the expression of genes related to phagocytosis and lipid metabolism. These completely activated microglial cells can only phagocytize Aβ, and may not cause damage to neurons and synapses. However, after Trem2 mutation or knock out, the expression of genes related to phagocytosis and lipid metabolism cannot be activated in microglial cells, which may cause microglial dysfunction, and thus these cells fail to protect neurons from Aβ induced injury. In addition, microglial cells may attack the synapses only the simultaneous presence of Aβ ad C1q. Whether the phenomenon in the present study is related to the Trem2 deficiency/mutation or C1q expression is still unclear. In our future studies, we will investigate the expression of Trem2, C11 and other genes related to phagocytosis and metabolism.
Our results showed Aβ could reduce REST expression only when its concentration equal to or higher than 10 µmol/L, which was accompanied by the reduced cellular activity. This suggests that, on one hand, high intracellular REST expression is neuroprotective, and on the other hand, REST at a high concentration may protect against inflammation induced by activated microglial cells. A variety of studies have confirmed that the REST expression in the nucleus of neurons in the prefrontal cortex and CA1 region of the hippocampus is positively related to the overall cognition, especially the short-term, semantic and working memory (5,9). REST may mediate cell death, antagonize stress and regulate the network related to the pathology of AD. In AD patients, REST expression is deficient in the nucleus of neurons, and the REST expression also reduces in the patients with mild cognitive disorder. However, the mechanism underlying the reduced REST in the nucleus is poorly understood. Our results showed the change in REST expression was opposite to that in CK1 expression in neurons after treatment with Aβ at different concentrations. That is, the CK1 expression increases, but REST expression reduces, which is accompanied by reduced neuronal activity. After use of CK1 inhibitor, the REST expression was reversed. In animal experiments, the change in REST expression was confirmed in neurons, especially that in the nucleus. CK1 is a member of serine/threonine protein kinase family and may phosphorylate the substrate protein at the C-terminal specific phosphorylation site to regulate the degradation of this substrate and affect the nuclear localization and gene expression (10). CK1 is a specific phosphorylase of REST and can phosphorylate REST at serine residue, which leads to the degradation of REST by SCFβ-TrCP ubiquitin ligase (11). Thus, we speculate that high Aβ load may up-regulate CK1 expression to increase REST phosphorylation, leading to its degradation by ubiquitination. In the following experiment, we will detect the expression of ubiquitinated REST in the brain of AD mice, aiming to explore the reason for deficiency of REST expression in the nucleus in the case of AD.
In conclusion, the plaques have been found in non-AD subjects and it is believed that AD patients are unlikely to be plaque-free. In addition, some old people with pathological features of AD may not develop dementia, which might be ascribed to the high nuclear REST expression. Thus, to investigate the mechanism underlying the reduced nuclear REST expression is helpful for the development of prophylactic interventions for asymptomatic subjects with pathological features of AD. High Aβ load may cause microglial cell dysfunction and loss of REST expression in the neurons, resulting in dementia. The reduced nuclear REST might be ascribed to its degradation by ubiquitination. This supports the hypothesis that high plaque load may increase the risk for dementia.
Acknowledgments
Funding: This study was supported by Shanghai Natural Science Foundation (No. 16ZR1426300).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by local ethics committee of Tenth People’s Hospital, Tongji University School of Medicine, Shanghai (No. SHDSYY-2016-2722). All the procedures were done according to the Guideline for Experimental Animal Use and Care as well as the Declaration of Helsinki. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Eikelenboom P, van Exel E, Veerhuis R, et al. Innate immunity and the etiology of late-onset Alzheimer's disease. Neurodegener Dis 2012;10:271-3. [Crossref] [PubMed]
- Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer's disease. Nat Immunol 2015;16:229-36. [Crossref] [PubMed]
- Liu YH, Zeng F, Wang YR, et al. Immunity and Alzheimer's disease: immunological perspectives on the development of novel therapies. Drug Discov Today 2013;18:1212-20. [Crossref] [PubMed]
- Sims R, van der Lee SJ, Naj AC, et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet 2017;49:1373-84. [Crossref] [PubMed]
- Lu T, Aron L, Zullo J, et al. REST and stress resistance in ageing and Alzheimer's disease. Nature 2014;507:448-54. [Crossref] [PubMed]
- Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918-34. [Crossref] [PubMed]
- Hong S, Beja-Glasser VF, Nfonoyim BM, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016;352:712-716. [Crossref] [PubMed]
- Vlad SC, Miller DR, Kowall NW, et al. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008;70:1672-7. [Crossref] [PubMed]
- Noh KM, Hwang JY, Follenzi A, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A 2012;109:E962-71. [Crossref] [PubMed]
- Kaneko N, Hwang JY, Gertner M. J Neurosci 2014;34:6030-9. [Crossref] [PubMed]
- Salminen A, Kaarniranta K, Haapasalo A, et al. Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer's disease. Prog Neurobiol 2012;96:87-95. [Crossref] [PubMed]


