
Clinical characteristics and management of cardiac and/or pulmonary cement embolus after percutaneous vertebroplasty: a single center experience
Introduction
Percutaneous vertebroplasty (PVP) is a therapeutic intervention that involves the injection of bone cement into a diseased vertebral body to stabilize the vertebrae and relieve back pain. This technique has been widely used to manage vertebral compression fractures (VCFs) because of its high efficacy and safety. However, in some cases, fatal complications such as cardiac and/or pulmonary cement embolus (CPCE) may occur as a result of bone cement leakage (1-3).
Currently, evidence regarding cement embolism complications is limited to few case reports, and guidelines for the management of such complications are unclear. Thus, this study analyzed the clinical characteristics and management of CPCE after PVP in VCFs to provide evidence for the management of this life-threatening complication.
Methods
Patient selection
Medical records (clinical characteristics and management) and imaging data (X-ray findings, CT findings, and fluoroscopic findings) of 12 patients with CPCE after PVP between October 2015 and July 2018 at our hospital were collected and examined retrospectively. Their health conditions were also evaluated through telephone follow-up survey. The study protocol was approved by the Zhejiang Taizhou Hospital institutional review board (K20181203) and conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). The need for informed consent was waived because of the retrospective nature of this study.
Clinical management
Case 1
A 70-year-old woman with VCFs of T10 and L4 underwent a successful PVP. She was asymptomatic after PVP. However, her spine X-ray (Figure 1A) and her chest and abdomen CT scan (Figure 1B) on post-PVP day 1 showed prevertebral cement leakage. She was solely managed with clinical observation due to the lack of symptoms. Unfortunately, CT pulmonary angiogram (CTPA) performed on post-PVP day 5 revealed a cardiac cement embolus of 5 cm long (Figure 1C) and a small pulmonary cement embolus (PCE) (Figure 1D). She was associated with a high risk of cardiac perforation because the tip of the cement embolus was inside the myocardium of the right atrium. Therefore, the cardiac cement embolus should be retrieved as soon as possible to avoid potentially catastrophic consequences.
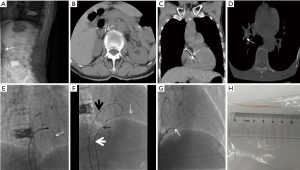
A multidisciplinary team was assembled to ensure an optimal treatment for the patient. Open heart surgery was initially planned to retrieve the cardiac cement. However, the patient was associated with surgical risk factors, such as age (70 years old) and diabetes history. Thus, a percutaneous procedure was recommended. The procedure was performed under electrocardiographic monitoring. A 6-F MPA2 catheter (Cordis Corporation, USA) was initially advanced percutaneously into the right atrium through the right femoral vein. A 5-F loop snare catheter (Shanghai Shape Memory Alloy Corporation, China) was manipulated to snare the cardiac cement. However, snaring the cement was impossible because the thread-like cement from the right atrium to the right ventricle did not have an accessible free end (Figure 1E).
A new vascular access was obtained to solve this dilemma by puncturing the right femoral vein and subsequently inserting an 8-F vascular sheath (Cordis Corporation, USA). An 8-F ablation catheter (Biosense Webster Ltd., Israel) was then introduced to relocate the cement, but this procedure also failed (Figure 1E). With this dilemma, the cement was attempted to be relocated via an innovative technique. A 0.035 inch guidewire (Terumo Corporation, Japan) and a 5-F TIG catheter (Terumo Corporation, Japan) were advanced into the right atrium via the 8-F vascular sheath. The guidewire was then manipulated to encompass the cement and pulled into the MPA2 catheter by the snare to form a loop. The loop was manipulated to slightly hook the cement. Subsequently, the cement was relocated, and an accessible free end was successfully released (Figure 1F). A 12-F long sheath (Lifetech Scientific Corporation, China) was then inserted and introduced to the right atrium by exchanging it with the previous 8-F vascular sheath. Afterward, the snare loop catheter was introduced and manipulated to snare the free end of the cement. The cement was successfully retrieved via the 12-F long sheath (Figure 1G). The retrieved cement was approximately 5 cm long (Figure 1H). The patient had a good post-procedure recovery and was discharged after 1 week.
Case 2
A 77-year-old woman underwent an uneventful PVP because of the VCF of L4. She was asymptomatic after PVP. However, an inferior vena cava (IVC) cement embolus (Figure 2A,B,C) and a small PCE (Figure 2C) were revealed by CT performed on postoperative day 1. She was immediately transferred to an angiography room for cement removal to avoid potentially catastrophic consequences. Percutaneous retrieval was performed under electrocardiographic monitoring.
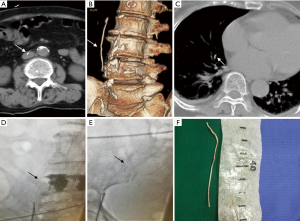
Vascular access was obtained by puncturing the right femoral vein and subsequently inserting a 10-F vascular sheath (Terumo Corporation, Japan). A 5-F loop snare catheter (Shanghai Shape Memory Alloy Corporation, China) was then manipulated to snare the free end of the IVC cement. Subsequently, the cement was snared and successfully retrieved via a 10-F vascular sheath (Figure 2D,E). The retrieved cement was approximately 7 cm long (Figure 2F). The patient had an uneventful post-procedure recovery and discharged after 5 days. The two patients were managed with close observation after percutaneous retrieval. The incidence of symptoms and physical signs such as coughing, hemoptysis, tachypnea, dyspnea, cyanosis, precordial chest pain or tightness, irregular cardiac rhythm, palpitation, dizziness, sweating, and hypotension was recorded during the follow-up period.
Conservative observation
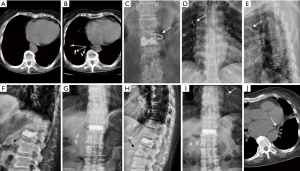
Ten patients with asymptomatic PCE diagnosed by X-ray and/or CT findings (Figures 3,4) were managed with conservative observation. During the follow-up period, the incidence of symptoms and physical signs such as coughing, hemoptysis, tachypnea, dyspnea, cyanosis, precordial chest pain or tightness, palpitation, dizziness, sweating, and hypotension was recorded.

Results
Clinical characteristics
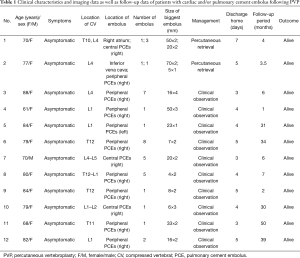
Twelve patients (mean age, 76.8±8.5 years; range, 61–88 years) with CPCE after PVP in VCFs were included in our study. Of these cases, 11 were female and 1 was male. All of the patients were asymptomatic (Table 1).
Full table
Imaging data
All of the patients with CPCE were diagnosed via X-ray and/or CT findings. One patient had combined cardiac and PCE; one patient had combined IVC and PCE. Of the 12 patients, 11 showed cement emboli located in the right pulmonary arteries, and 9 manifested peripheral embolisms. The number of PCE varied from 1 to 8 (Table 1).
Management result and follow-up
Two patients were successfully treated with a percutaneous retrieval procedure and discharged after 7 and 5 days. Ten asymptomatic PCE patients were managed with conservative observation and discharged 3–5 days later. All of the patients were alive and remained asymptomatic during the follow-up period (median, 6.5 months; range, 1–50 months) (Table 1).
Discussion
Although PVP is a minimally invasive and relatively safe procedure, the number of PVP-related complications has increased because of the widespread application of PVP. The complications that have been reported include pain, infection, inaccurate needle placement, hemorrhage at puncture sites, cement leakage into the spinal canal, perivertebral tissue leakage, perivertebral venous leakage, pulmonary cement embolism, and cardiac cement embolism (4,5).
CPCE, which is caused by cement leakage migrating from the perivertebral venous to the IVC, right heart, and pulmonary arteries, is the most fatal complication. Most patients with CPCE are asymptomatic (6,7), and only a small percentage (0.4% to 0.9%) of patients may manifest chest pain, coughing, dyspnea, hemoptysis, syncope, acute respiratory distress, and cardiac tamponade (8,9). Similar to observations in other reported studies, our findings revealed that all of the patients with CPCE during the follow-up period remained asymptomatic.
The incidence of PCE is not well documented and appears to be undervalued. Chest X-ray and CT examinations are two common imaging methods. Many patients may not undergo post-PVP chest X-ray or CT examination because most PCE cases are asymptomatic. Therefore, missed diagnosis cannot be avoided. Varied imaging methods (X-ray or CT) may also contribute to the undervalued incidence of PCE. The incidence of PCE is as low as 3.5% and may reach 28.6% (10). The incidence varies from 1% to 6.8% when only postprocedural chest X-ray is employed (11). However, if sensitive examination such as chest CT is performed, the incidence dramatically increases from 2.1% to 26% (12,13), suggesting that the incidence of PCE may be undervalued if postprocedural chest X-ray is used as the diagnostic modality. In some instances, differentiating PCE from calcifications on chest CT images is difficult, and such a difficulty could lead to misdiagnosis. PCE, unlike pulmonary thromboembolism, has a higher density than lung parenchyma and can be easily recognized on a chest X-ray and CT. However, radiologists should be aware that calcifications are also high in density and can be difficult to differentiate from PCE in some cases. Thus, radiologists should combine a patient’s PVP history and make a cautious diagnosis. The presence of high-attenuation signals in the lumen of the pulmonary artery is highly suggestive of PCE (14). Comparison with a preprocedure chest radiograph or CT is important for the diagnosis of PCE because a newly developed branching high-attenuation signal in the pulmonary artery after PVP should be considered as PCE. Three cases of patients (cases 3, 5, and 8) in our study were diagnosed as PCE through a comparison with pre-procedure imaging.
Cardiac cement embolus and PCE should be dealt with differently because the varied severity of these two entities. Currently, the best treatment for PCEs remains controversial and poorly characterized. Krueger et al. (15) theorized that the location of the cement and the severity of symptoms are two major considerations for the appropriate treatment of PCEs. Therefore, careful observation is recommended for asymptomatic peripheral PCEs. Careful observation and anticoagulation are recommended for symptomatic peripheral or asymptomatic central embolisms. Surgical treatment is recommended for symptomatic central embolisms. However, the benefits of anticoagulation for asymptomatic PCEs remain controversial. Anticoagulation is necessary to reduce the risk of thrombus formation on cement. However, no evidence exists to show the formation of thrombus on the cement. On the contrary, Blinc et al. (16) performed an in vitro study and demonstrated that the surface of fresh or old cement does not accelerate platelet aggregation or plasma coagulation. In addition, patients with PCE received no anticoagulation therapy and remained asymptomatic during long-term follow-up (17,18), indicating that asymptomatic PCEs may be effectively managed with careful observation. Our results also demonstrated that patients with asymptomatic peripheral or central embolisms could be safely managed with careful observation and clinical follow-up.
Cardiac cement embolus must be removed because of the risk of cardiac rupture and cardiac tamponade (4). Open heart surgery and percutaneous procedure are two feasible options for the retrieval of cardiac cement embolus (19). Our initial plan involved an open-heart surgery to retrieve the cardiac cement embolus. However, given the high surgical risk, poor prognosis, and diabetes history of the elderly patient (70 years old), percutaneous procedure was recommended. However, the percutaneous procedure in the present case was eventful because the cement embedded into the lateral wall of the right atrium. Many methods were conducted, and the cement was finally retrieved with a modified wire-loop snare technique. Although the cardiac cement embolus was successfully retrieved via the percutaneous procedure in the present study, the percutaneous procedure, requiring highly skilled techniques, remained challenging and dangerous because the shape and size of the cardiac cement embolus in different patients might vary. The percutaneous procedure is less traumatic than an open-heart surgery. However, the percutaneous procedure is more dangerous than the open procedure. In some cases, a complete percutaneous procedure is impossible. Therefore, open heart surgery should be considered as the preferred treatment, and percutaneous procedure can be used as alternative treatment for patients with surgical contraindication or other surgical high-risk factors.
Lee et al. (20) described a patient who developed an IVC cement spike after PVP was treated conservatively because the patient was asymptomatic. This patient died of acute respiratory distress syndrome 3 months later because of a cardiac tamponade caused by the cement spike that pierced the right ventricle. This study indicated that the watch-and-wait policy for IVC cement spikes should not be recommended because of fatal cardiac tamponade. A patient (case 5) in our study developed an IVC cement spike following PVP and was also treated conservatively because the patient was asymptomatic. The post-procedure chest X-ray and CT performed 1 month later revealed that the IVC cement spike broke and migrated into the segmental pulmonary arteries of the left inferior lobe. Fortunately, a serious cardiac event, such as a cardiac perforation or cardiac tamponade, did not occur in this case. Similar to this case, patient 2 with an early percutaneous retrieval of an IVC cement spike had a good patient outcome. Our results demonstrated that the early retrieval of an IVC cement spike could be recommended for patients with IVC cement spike to avoid a potentially fatal complication.
Conclusions
The incidence of PCE caused by cement leakage following PVP appears to be undervalued. More attention should be paid to the possibility of development of CPCE. For patients with cardiac cement embolus, open heart surgery should be considered as the preferred treatment. For patients with surgical contraindications or other surgical high-risk factors, percutaneous procedure could be used as an alternative treatment. For patients with an IVC cement spike, percutaneous retrieval procedure should be recommended. Careful observation and clinical follow-up should be recommended for patients with asymptomatic peripheral or central embolisms.
Acknowledgments
Funding: This work was partially supported by the Taizhou Science and Technology Agency, China (1701KY25).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Zhejiang Taizhou Hospital institutional review board (K20181203) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
References
- Venmans A, Lohle PN, van Rooij WJ, et al. Frequency and outcome of pulmonary polymethylmethacrylate embolism during percutaneous vertebroplasty. AJNR Am J Neuroradiol 2008;29:1983-5. [Crossref] [PubMed]
- Sinha N, Padegal V, Satyanarayana S, et al. Pulmonary cement embolization after vertebroplasty, an uncommon presentation of pulmonary embolism: A case report and literature review. Lung India 2015;32:602-5. [Crossref] [PubMed]
- Caynak B, Onan B, Sagbas E, et al. Cardiac tamponade and pulmonary embolism as a complication of percutaneous vertebroplasty. Ann Thorac Surg 2009;87:299-301. [Crossref] [PubMed]
- Park JH, Choo SJ, Park SW. Images in cardiovascular medicine. Acute pericarditis caused by acrylic bone cement after percutaneous vertebroplasty. Circulation 2005;111:e98. [Crossref] [PubMed]
- Cortet B, Cotten A, Boutry N, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures: an open prospective study. J Rheumatol 1999;26:2222-8. [PubMed]
- Kim YJ, Lee JW, Park KW, et al. Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: incidence, characteristics, and risk factors. Radiology 2009;251:250-9. [Crossref] [PubMed]
- Groen RJ, du Toit DF, Phillips FM, et al. Anatomical and pathological considerations in percutaneous vertebroplasty and kyphoplasty: a reappraisal of the vertebral venous system. Spine (Phila Pa 1976) 2004;29:1465-71. [Crossref] [PubMed]
- Lee IJ, Choi AL, Yie MY, et al. CT evaluation of local leakage of bone cement after percutaneous kyphoplasty and vertebroplasty. Acta Radiol 2010;51:649-54. [Crossref] [PubMed]
- Medical Advisory Secretariat. Percutaneous vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: an evidence-based analysis. Ont Health Technol Assess Ser 2010;10:1-45. [PubMed]
- El Saman A, Kelm A, Meier S, et al. Intraoperative PEEP-ventilation during PMMA-injection for augmented pedicle screws: improvement of leakage rate in spinal surgery. Eur J Trauma Emerg Surg 2013;39:461-8. [Crossref] [PubMed]
- Bliemel C, Buecking B, Struewer J, et al. Detection of pulmonary cement embolism after balloon kyphoplasty: should conventional radiographs become routine? Acta Orthop Belg 2013;79:444-50. [PubMed]
- Anselmetti GC, Corgnier A, Debernardi F, et al. Treatment of painful compression vertebral fractures with vertebroplasty: results and complications. Radiol Med 2005;110:262-72. [PubMed]
- Venmans A, Klazen CA, Lohle PN, et al. Percutaneous vertebroplasty and pulmonary cement embolism: results from VERTOS II. AJNR Am J Neuroradiol 2010;31:1451-3. [Crossref] [PubMed]
- Trumm CG, Pahl A, Helmberger TK, et al. CT fluoroscopy-guided percutaneous vertebroplasty in spinal malignancy: technical results, PMMA leakages, and complications in 202 patients. Skeletal Radiol 2012;41:1391-400. [Crossref] [PubMed]
- Krueger A, Bliemel C, Zettl R, et al. Management of pulmonary cement embolism after percutaneous vertebroplasty and kyphoplasty: a systematic review of the literature. Eur Spine J 2009;18:1257-65. [Crossref] [PubMed]
- Blinc A, Bozic M, Vengust R, et al. Methyl-methacrylate bone cement surface does not promote platelet aggregation or plasma coagulation in vitro. Thromb Res 2004;114:179-84. [Crossref] [PubMed]
- Choe DH, Marom EM, Ahrar K, et al. Pulmonary embolism of polymethyl methacrylate during percutaneous vertebroplasty and kyphoplasty. AJR Am J Roentgenol 2004;183:1097-102. [Crossref] [PubMed]
- Mansour A, Abdel-Razeq N, Abuali H, et al. Cement pulmonary embolism as a complication of percutaneous vertebroplasty in cancer patients. Cancer Imaging 2018;18:5. [Crossref] [PubMed]
- Hatzantonis C, Czyz M, Pyzik R, et al. Intracardiac bone cement embolism as a complication of vertebroplasty: management strategy. Eur Spine J 2017;26:3199-205. [Crossref] [PubMed]
- Lee V, Patel R, Meier P, et al. Conservative management of inferior vena cava cement spike after percutaneous vertebroplasty causes fatal cardiac tamponade. J Rheumatol 2014;41:141-2. [Crossref] [PubMed]


