
The assessment of combined karyotype analysis and chromosomal microarray in pregnant women of advanced maternal age: a multicenter study
Introduction
In recent years, Chinese fertility policy has undergone major reforms. Open two children policy was full implementation in China from 2016. This has been accompanied by a sharp increase in the number of pregnant women at advanced maternal age (AMA) (1). According to a Chinese survey, the proportion of AMA was 10.1% in 2011 (2), and it increased to 20.5% in 2016 (3). It has been recognized that women at AMA would have adverse effects both to mothers and infants, such as decreased fertility, increased spontaneous abortion rate, fetal chromosomal abnormalities, hypertensive complications and increased incidence of intrauterine fetal death, etc. (4-7). Particularly, AMA women will face an increased risk of birth defects (8). Faced with the growing number of AMA women, the situation of birth defects prevention is extremely severe in China.
Until now, there was no unified plan for effectively prenatal screening and diagnosis for women at AMA. Most countries regard AMA as an indicator of interventional prenatal diagnosis, including amniocentesis (AC) and chorionic villus sampling (CVS). For example in China, we usually first recommend interventional prenatal diagnosis to the AMA pregnant women who come to the hospital for genetic counseling. However, more and more experts have questioned whether consider age as the only indication appropriate? Some countries have changed the strategy of prenatal screening and diagnosis for AMA women (9). American Association of Obstetricians and Gynecologists (ACOG) (10) suggested that all the pregnant women could accept serological prenatal screening whether their age was over 35 years old or not. Meanwhile, noninvasive prenatal screening (NIPS) was regarded as a good choice for AMA pregnant women. It could not only achieve satisfactory clinical effect, but also greatly reduce invasive prenatal diagnosis (11). AMA women were also willing to accept it and became the largest population for NIPS testing (12). But it still had some limitations. About 12.4% of fetal chromosomal abnormalities would be missed if NIPS completely replaced invasive prenatal diagnosis in AMA women (13). According to a retrospective cross-sectional survey (14), AMA represents an absolute indication for invasive tests appears deeply rooted.
All the time, standard karyotype analysis is regarded as the traditional method for the prenatal diagnosis, which can detect major chromosomal abnormalities, such as aneuploidy, unbalanced rearrangements, Robertsonian translocation, mosaicism. Recently, chromosomal microarray (CMA), as a high resolution genomic technology, was also applied in prenatal diagnosis of genetic disorders. It offers additional diagnostic benefits by revealing sub-microscopic imbalances or copy number variations (CNVs) which can’t be detect by standard karyotype analysis (15,16). In samples with a normal karyotype, CMA revealed pathogenic and potential for clinical significance in 1.7% of those whose indications were AMA (17).
In present study, the clinical data of 4,224 women at AMA who accepted prenatal diagnosis were collected from two prenatal diagnosis centers. They received the detection of traditional karyotype analysis and/or CMA. We analyzed the results and hoped to provide scientific clinical guidance to receive prenatal diagnosis for AMA women.
Methods
Patients and design
The study design and protocol were reviewed and approved by the ethics committee of Changzhou Maternity and Child Health Care Hospital Affiliated to Nanjing Medical University (No. 2017003). All pregnant women received genetic counseling and signed a written consent before the test.
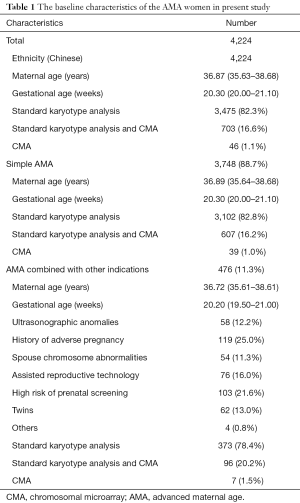
From February 2015 to November 2018, 4,224 women at AMA (over 35 years old at delivery) who accepted prenatal diagnosis by AC were recruited for this study. They were from two prenatal diagnosis centers: Changzhou Maternity and Child Health Care Hospital affiliated to Nanjing Medical University and The Affiliated Suzhou Hospital of Nanjing Medical University. Most of the pregnant women accepted prenatal diagnosis because of the indication of AMA. But some AMA women also combined with other indications, such as ultrasonographic anomalies, history of adverse pregnancy, spouse chromosome abnormalities, and so on. They were 35–46 years old and their gestational weeks were 15–25 w. Table 1 showed the baseline characteristics of all AMA women.
Full table
Both centers used the same detection platform, experimental scheme, and quality control standards. They all participated in the laboratory quality control evaluation plan.
Prenatal diagnosis
The experimental methods of cytogenetic prenatal diagnosis were similar to our previous reports (11). After AC, two individuals independently completed the cell karyotype test of prenatal diagnosis. We used two sets of culture system: CHANG Amnio (Emergo Europe Prinsessegracht 20 2514 AP The Hague, The Netherlands) and Amniotic fluid cell culture medium (Hangzhou Baorong Science and Technology Ltd). We used GSL-120 (Leica Biosystems Richmond, Inc.) for karyotypes scanning and software (CytoVision Automated Cytogenetics Platform) for chromosome karyotypes analysis. At least five cell karyotypes were analyzed and 20 karyotypes were counted. Sixty to one hundred karyotypes were counted for the cases with chromosome mosaicism.
CMA analysis
From Jan 2015, we applied CMA in prenatal diagnosis for the pregnant women with demand and willingness to accept the test. Amniotic fluid (10 mL) was collected with informed consents. Genomic DNA was extracted using a QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA, USA). The DNA (250 ng) was amplified, labeled, and hybridized to GCS 3000Dx v.2 platform (Affymetrix, USA) according to the manufacturer’s protocol. SNP array test was performed using a commercial 750K microarray chip (Affymetrix CytoScan 750K Array). After hybridization with fragmented DNA, the chip was washed with buffer and scanned by Alaser scanner. The data was analyzed with the use of Chromosome Analysis Suite v3.2 (ChAs) software package.
Statistical analysis
The data were analyzed using EmpowerStats software (X&Y Solutions, Inc.) and R (http://www.R-project.org) (18). The parameters of age and gestational age were expressed as median (M), 2.5th percentile (P2.5) and 97.5th percentile (P97.5). χ2 test were used to compare proportions between two groups. The Chi-square test was used to compare the counting data. The increasing trend was analyzed by Cochran-Armitage test. P<0.05 was chosen to be statistically significant.
Results
Within the period, 4,224 women at AMA accepted prenatal diagnosis in two centers. Of them, 3,748 women were simple AMA and 476 women combined with other indications, such as prenatal ultrasound abnormalities, adverse pregnancy history, couple chromosome abnormalities, assisted reproductive technology pregnancy, etc. (Table 1). In total, 3,475 (82.3%) women received karyotype analysis only, 703 (16.6%) were examined by both karyotype analysis and CMA, while 46 cases selected CMA only. Recently, more and more AMA women were willing to select CMA technology. In 2015, only 1% of pregnant women would receive additional CMA tests. But 10.3% in 2016, 26.4% in 2017 and 58.0% of AMA women in 2018 would choose this technology. Meanwhile, when the AMA women have other abnormal manifestations, they were also more willing to choose CMA. The ratio was about 21.6% (103/476), which was higher than it of simple AMA women (21.6% vs. 17.2%, P=0.018).
In our study, a total of 164 women with fetal chromosomal abnormal results were detected, the abnormality rate was 3.88% (164/4,224). Among them, 145 (3.4%, 145/4,224) women were detected as abnormal chromosome number, 19 cases (0.4%, 19/4,224) as abnormal chromosome structure. Trisomy 21 syndrome (T21) was the most common type of abnormal chromosome number. We found a total of 87 pregnant women carried with T21 fetus. In addition, trisomy 18 (T18) and fetal sex chromosome aneuploidy (SCAs) were also common. Balanced translocation was the most common type of chromosomal structural abnormalities. Compared with simple AMA women, the abnormality rate was significantly increased in the AMA women combined with other indications, particularly in chromosomal number abnormalities (22.5% vs. 1.0%, P<0.001, Table 2). Meanwhile, a total of 48 CNVs were detected in this study. Among them, 10 cases (0.24%, 10/4,224) were proved as pathogenic or likely pathogenic CNVs. However, there was no significant difference in abnormality rate between the two groups (9/3,748 vs. 1/476, P=0.395, Table 2). Among 476 AMA women combined with other indications, the chromosome number abnormality rate of high-risk group after prenatal screening was the highest, which followed by the pregnant women with abnormal prenatal ultrasound (Table 3). However, when the couples had chromosomal abnormalities, the risk of chromosomal structural abnormalities was increased (Table 3).

Full table

Full table
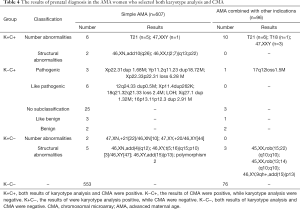
Seven hundred and three AMA women received both karyotype analysis and CMA test in present study. Their results were showed in Table 4. With the CMA technology, we could find 46 cases with CNVs in addition, although most of them were benign, like benign or no subclassification. Only 10 CNVs were pathogenic or likely pathogenic. For the AMA pregnant women, the rate of additional abnormalities with clinical significance by CMA was 1.42% (10/703). However, 10 cases with chromosomal abnormalities (mainly were structural abnormalities) would be missed if CMA was used alone. Few AMA pregnant women chose CMA alone for prenatal diagnosis. In this study, three abnormalities were found in 46 pregnant women, including 1 case of 47,XXY, 2 cases with no subclassification CNVs.
Full table
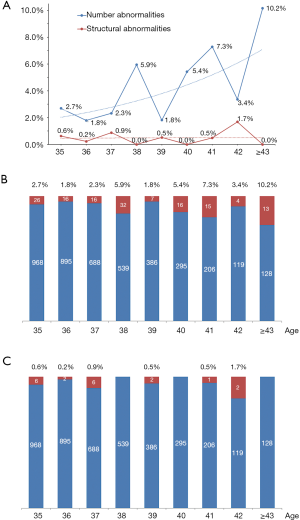
Figure 1 showed the relationship between the age of AMA women and the chromosome abnormality rate. After subgroup analyzed by age, the increasing trend of abnormalities rate with age was analyzed by Cochran-Armitage test, and alternative hypothesis was “increasing trend”. We found that chromosome number abnormalities significantly increased with age (P<0.001), while there were no such trends in chromosomal structural abnormalities (P=0.624) (Table 5). Because of too few cases of pathogenic or like pathogenic CNVs, we did not make a trend analysis.

Full table
In addition, we found a total of 76 women with chromosome polymorphism with karyotype analysis, mainly including pericentric inversion, chromosome constriction. Among them, 14 cases also received CMA test. However, none was detected as pathogenic or likely pathogenic CNVs. Meanwhile, through followed up of pregnancy outcomes of these women, no serious abnormalities in newborns have been found so far.
Discussion
There were great difference of the strategies of prenatal screening and diagnosis for pregnant woman in advanced age between different countries. Some countries advocate prenatal screening for all pregnant women, regardless of their age (10). In China, the current strategy is to offer invasive prenatal procedures. They can also choose NIPS prudently when they refuse prenatal diagnosis. With the increase of the number of AMA pregnant women in China, the pressure of prenatal diagnosis is still enormous. Despite the increasing availability and effectiveness of NIPS and it decreased the rate of AC (19), most AMA women still opt for invasive tests (14). At the same time, the prenatal diagnosis technology is also undergoing tremendous changes. Based on the traditional karyotype analysis, more and more pregnant women are willing to accept additional molecular prenatal diagnosis technology. Facing these new changes, further in-depth analysis of clinical data of prenatal diagnosis of AMA pregnant women will help to improve the strategies and reasonable select appropriate technologies. This is a four-year clinical study from two prenatal diagnosis centers involving 4,224 clinical samples. As our results show, most pregnant women in AMA choose both traditional karyotype analysis and CMA for prenatal diagnosis, especially when they combined with other abnormal indications.
The women at AMA will face an increased risk of fetal chromosomal disorder. According our results, 3.88% AMA women carried the fetuses of chromosomal abnormalities, which were similar to other reports (13,20,21), but higher than other group’s reports (8). However, it was consistent that the abnormality rate increased with the age of AMA pregnant women (22,23). Our study further found that this increase was only reflected in chromosome number abnormalities. Chromosome structural abnormalities did not increase with the mother’s age. At the same time, our results also suggested that the rate of fetal chromosomal abnormalities could increase significantly when his mother have other abnormal indications. Therefore, it is particularly necessary to emphasize the importance of prenatal diagnosis for some AMA pregnant women, especially when she combined with high risk of prenatal screening and spouse chromosome abnormalities, and/or her age is old enough.
In prenatal diagnosis, CMA has considerable diagnostic and prognostic values, but has not yet fully replaced karyotype analysis. It is well known that CMA, as a high resolution genomic technology, can detect sub-microscopic imbalances or CNVs which traditional karyotype analysis can’t detect (15). There were lots of studies reported the added detection of pathogenic abnormalities with CMA in comparison to the traditional karyotyping, especially in fetuses with multiple or isolated ultrasound abnormalities (24,25). It is believed that the application of CMA in prenatal diagnosis can increase the diagnostic rate by about 1–6% (15,26,27). However, few studies have been devoted to the clinical application of CMA in AMA pregnant women. Wapner’s group reported that microarray analysis revealed clinically relevant deletions or duplications in 1.7% of those whose indications were AMA in samples with a normal karyotype (17). Their results were similar to us. We found the rate of additional abnormalities with clinical significance by CMA was 1.42%. However, it was amazing that there was no relationship between the AMA women whether or not combined with other indications. This might be related to our smaller clinical sample size. Further accumulation of clinical data and further analysis according to different abnormal indications may be helpful to fully elaborate the application value of CMA technology in the field of prenatal diagnosis of AMA pregnant women. On the other side, we also found some chromosomal abnormalities would be missed if CMA was used alone. Fortunately, most of them were structural abnormalities and not fateful. But these abnormalities may bring the risk of infertility and abortion. Therefore, it is also inappropriate that CMA completely replace to karyotyping in prenatal diagnosis. In addition, we found chromosome polymorphism were also common in prenatal diagnosis with karyotype analysis, mainly including pericentric inversion, chromosome constriction. After followed up their pregnancy outcomes, no serious abnormalities in newborns have been found so far. However, these pregnant women still had varying degrees of anxiety and tension. The application of CMA can determine whether these polymorphisms have clinical significance. It can contribute to prenatal consultation of clinicians and greatly reduce these mothers’ tension.
In conclusion, we retrospectively analyzed the results of prenatal diagnosis for AMA pregnant women from two centers. More and more pregnant women in AMA were willing to accept traditional karyotyping combined with CMA, particularly when the women had other abnormal indications. About 3.88% fetuses of AMA women had chromosomal abnormalities, the abnormality rate increased with their age. This increase was only reflected in chromosome number abnormalities, but not in structural abnormalities. The application of CMA could increase the diagnostic rate by about 1.4% for AMA women, and greatly reduce their tension.
Acknowledgments
We thank all the project participants for their contributions.
Funding: This study was funded by Project supported by National Natural Science Foundation of China (No. 81773438), Key Research and Development Plan Project of Jiangsu Province (BE2017650), Suzhou Key Medical Center (SZZX201505), Jiangsu Provincial Medical Innovation Team (CXTDB2017013), Suzhou Clinical Medical Expert Team (SZYJTD201708), Changzhou Science and Technology Support Project (Social Development CE20175021) and Jiangsu Maternal and Children Health Care Key Discipline (FXK201748, FXK201754).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study design and protocol were reviewed and approved by the ethics committee of Changzhou Maternity and Child Health Hospital affiliated to Nanjing Medical University (No. 2017003). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Xie M, Lao TT, Du M, et al. Risk for Cesarean section in women of advanced maternal age under the changed reproductive policy in China: A cohort study in a tertiary hospital in southwestern China. J Obstet Gynaecol Res 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Liu X, Zou L, Chen Y, et al. Effects of maternal age on pregnancy:a retrospective cohort study. Zhonghua Yi Xue Za Zhi 2014;94:1984-8. [PubMed]
- Chen Y, Zheng XL, Wu SW, et al. Clinic characteristics of women with advanced maternal age and perinatal outcomes. Zhonghua Fu Chan Ke Za Zhi 2017;52:508-13. [PubMed]
- Heffner LJ. Advanced maternal age--how old is too old? N Engl J Med 2004;351:1927-9. [Crossref] [PubMed]
- Velazquez MA, Smith CG, Smyth NR, et al. Advanced maternal age causes adverse programming of mouse blastocysts leading to altered growth and impaired cardiometabolic health in post-natal life. Hum Reprod 2016;31:1970-80. [Crossref] [PubMed]
- Lisonkova S, Potts J, Muraca GM, et al. Maternal age and severe maternal morbidity: A population-based retrospective cohort study. PLoS Med 2017;14:e1002307. [Crossref] [PubMed]
- Cakmak Celik F, Aygun C, Kucukoduk S, et al. Maternal and neonatal outcomes in advanced maternal age: a retrospective cohort study. J Matern Fetal Neonatal Med 2017;30:2452-6. [Crossref] [PubMed]
- Zhu Y, Lu S, Bian X, et al. A multicenter study of fetal chromosomal abnormalities in Chinese women of advanced maternal age. Taiwan J Obstet Gynecol 2016;55:379-84. [Crossref] [PubMed]
- Nakata N, Wang Y, Bhatt S. Trends in prenatal screening and diagnostic testing among women referred for advanced maternal age. Prenat Diagn 2010;30:198-206. [PubMed]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 88, December 2007. Invasive prenatal testing for aneuploidy. Obstet Gynecol 2007;110:1459-67. [Crossref] [PubMed]
- Yu B, Lu BY, Zhang B, et al. Overall evaluation of the clinical value of prenatal screening for fetal-free DNA in maternal blood. Medicine (Baltimore) 2017;96:e7114. [Crossref] [PubMed]
- Yu B, Li H, Chen YP, et al. Clinical evaluation of NIPS for women at advanced maternal age: a multicenter retrospective study. J Matern Fetal Neonatal Med 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Chen YP, He ZQ, Shi Y, et al. Not all chromosome aberrations can be detected by NIPT in women at advanced maternal age: A multicenter retrospective study. Clin Chim Acta 2018;486:232-6. [Crossref] [PubMed]
- Godino L, Pompilii E, D'Anna F, et al. Attitudes of women of advanced maternal age undergoing invasive prenatal diagnosis and the impact of genetic counselling. Eur J Hum Genet 2016;24:331-7. [Crossref] [PubMed]
- Levy B, Wapner R. Prenatal diagnosis by chromosomal microarray analysis. Fertil Steril 2018;109:201-12. [Crossref] [PubMed]
- Ganapathi M, Nahum O, Levy B. Prenatal Diagnosis Using Chromosomal SNP Microarrays. Methods Mol Biol 2019;1885:187-205. [Crossref] [PubMed]
- Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med 2012;367:2175-84. [Crossref] [PubMed]
- Yu B, Long W, Yang Y, et al. Newborn Screening and Molecular Profile of Congenital Hypothyroidism in a Chinese Population. Front Genet 2018;9:509. [Crossref] [PubMed]
- Kim SM, Kim HH, Han YJ, et al. Change in rates of prenatal tests for chromosomal abnormality over a 12-year period in women of advanced maternal age. Obstet Gynecol Sci 2018;61:453-60. [Crossref] [PubMed]
- Ocak Z, Özlü T, Yazıcıoğlu HF, et al. Clinical and cytogenetic results of a large series of amniocentesis cases from Turkey: report of 6124 cases. J Obstet Gynaecol Res 2014;40:139-46. [Crossref] [PubMed]
- Xiao H, Yang YL, Zhang CY, et al. Karyotype analysis with amniotic fluid in 12365 pregnant women with indications for genetic amniocentesis and strategies of prenatal diagnosis. J Obstet Gynaecol 2016;36:293-6. [Crossref] [PubMed]
- Ma J, Hong P, Fu J, et al. Prenatal diagnostic testing among women referred for advanced maternal age in Beijing, 2001-2012. Int J Gynaecol Obstet 2014;125:232-6. [Crossref] [PubMed]
- Kim YJ, Lee JE, Kim SH, et al. Maternal age-specific rates of fetal chromosomal abnormalities in Korean pregnant women of advanced maternal age. Obstet Gynecol Sci 2013;56:160-6. [Crossref] [PubMed]
- Oneda B, Rauch A. Microarrays in prenatal diagnosis. Best Pract Res Clin Obstet Gynaecol 2017;42:53-63. [Crossref] [PubMed]
- Wang Y, Cao L, Liang D, et al. Prenatal chromosomal microarray analysis in fetuses with congenital heart disease: a prospective cohort study. Am J Obstet Gynecol 2018;218:244.e1-17. [Crossref] [PubMed]
- Mademont-Soler I, Morales C, Soler A, et al. Prenatal diagnosis of chromosomal abnormalities in fetuses with abnormal cardiac ultrasound findings: evaluation of chromosomal microarray‐based analysis. Ultrasound Obstet Gynecol 2013;41:375-82. [Crossref] [PubMed]
- Breman A, Pursley AN, Hixson P, et al. Prenatal chromosomal microarray analysis in a diagnostic laboratory; experience with >1000 cases and review of the literature. Prenat Diagn 2012;32:351-61. [Crossref] [PubMed]


