
Turning stem cells homing potential into cancer specific drug delivery machines
Early diagnosis is essential in the successful treatment of prostate cancer. The remission rate of metastasized prostate cancer presents a lifelong challenge for the patients. Therefore molecular research to identify potential therapeutic targets for advanced prostate cancer is crucial to increase the lifespan of patients and reduce remission rates. In this issue of Stem Cells Translational Medicine, Michael T. Schweizer et al. test the homing efficiency of mesenchymal stem cell (MSC) in men with localized prostate cancer. After systemically infused allogenic MSC’s prostatectomy was conducted within 6 days. Schweizer et al. firstly observed that the infusions of MSC’s are safe and secondly allogenic MSC’s did not localize within the prostate cancer loci. The importance of these results is that the systemic delivery of bone marrow mesenchymal stem cells (BM-MSCs) alone, without specific targeting to the tumor site, might not influence the homing potential of BM-MSCs and their potential as a therapeutic vehicle.
MSC homing potential and associated risks
MSCs are shown to present homing potential to site of injury and tumor in animal models and have potential to be used as delivery vehicles for therapeutic agents to the tumor sites (1). Although the homing mechanism of MSCs to the tumor site is not fully understood, the de facto MSC homing to the tumor site not in sufficient numbers to induce the restoration of organ function through regeneration.
The chemokines and interleukins secreted by MSCs might induce migration, angiogenesis and immunosuppression to contribute tumor stroma (2,3). Furthermore, MSCs might also contribute to the tumor micro-environment with their differentiation potential and possibly promote epithelial-mesenchymal transition (EMT) to induce metastasis at primary tumor sites (4). The self renewal potential of MSCs also introduces additional growth and survival factors to the tumor micro-environment. In addition to this, promotion of MSCs might also induce secondary tumors in a failed attempt to restore tissue function (5). Therefore the risks associated with MSC differentiation potential should be evaluated thoroughly before application of MSC based therapies in human cancers.
Cancer therapy is a tall task given that the cancer cells have impaired self renewal, differentiation and growth potential. Targeted cancer therapy is even trickier as the identification of tumor cells is necessary in vivo without suppressing apoptosis and inducing proliferation in tumor cells while conserving the health and function of the fully differentiated and mature somatic cells.
Schweizer et al. 2019 used BM-MSCs to asses homing ability and safety of BM-MSCs in prostate cancer patients. The author’s argued that first line of therapy to prostate cancer metastasis is only successful in the initial phase of the treatment to be followed by further expansion of the prostate cancer and potential to be lethal. Several non-targeted chemotherapeutic drugs may increase the survival rate of the patients; however they are far from optimal benefit for the patients. MSCs are able to home to prostate cancer sites due to presence of cytokines and chemokines in tumor microenvironment in prostate cancer similar to other cancers (6). Therefore use of MSCs as targeting vehicles to deliver drugs to the prostate cancer site would be potential approach towards targeted therapy in prostate cancer. Authors have applied this idea as phase 1 trials since human in vivo evidence is not yet sufficient.
BM-MSCs as delivery vehicles
Schweizer et al. 2019 used related and oriented methods to assess the homing potential and the safety of BM-MSCs in prostate cancer patients. The inclusion criteria for patients were in parallel with the research intentions. The samples of prostate cancer patients were collected after previously determined surgeries and allogenic infusions of MSCs to the patients eliminating ethical concerns. Patients with autoimmune disease and antibiotics were excluded as it can change the homing pattern and distort the expansion of MSCs. Following sample retention from the patients the MSCs were separated and expanded in parallel with international standards. The MSC infusion and follow up period was 28 days for the initial cohort and 4 to 6 days for full cohort.
The authors used a version of digital PCR to quantify and detect BM-MSC DNA in prostate cancer samples. In short, the relative quantification of donor DNA and patient DNA was measured in prostate cancer sites where the homing of BM-MSCs was expected. Six SNPs were used to distinguish between donor and patient DNA whose SNP profiles were previously established. Authors reported that there were no detectable homing of MSCs to the primary tumor sites followed their study with a HLA locus containing 18 SNPs which also did not show any distinguishable patient and donor DNA.
Possible reasons for non-homing of BM-MSCs to primary prostate cancer sites
The results presented by Schweizer et al. 2019 show that the systemically administered BM-MSCs did not home to the primary prostate cancer sites. One of the possible reasons behind this result was BM-MSCs do not actually home to the primary prostate cancer sites without proper signaling. MSC are known to home to sites with inflammation and tumors generate an inflammatory signal that can trigger MSC homing. However in this study, allogenic MSCs might have not responded to the inflammatory signal generated by the prostate tumors. Although allogenic MSCs should not be HLA matched and reportedly well tolerated by patients in this study, failure to home the prostate tumor sites by allogenic BM-MSCs should question their ability to target cancer cells without reprogramming. We have previously proposed a possible way to reprogram MSCs to specifically target tumor sites genetic reprogramming using a suicide gene to target telomerase active cells (7). Tumor cells usually activate telomerase to bypass the Hayflick limit and have continuous proliferation (8). Activation of telomerase is also a critical step in metastatic transformation as tumor cell mass should increase significantly, generating hypoxia before activation of angiogenic factors such as vascular endothelial growth factor (VEGF). Subsequently, within reach to circulation, tumor cells may undergo EMT to metastasize distant organs (9). The allogenic MSCs would be recruited to the tumor site only after the autologous MSCs were recruited to contribute to inflammation, angiogenesis and EMT.
Targeting telomerase active tumor cells with MSCs carrying suicide gene might prove to be a substantial approach to selectively induce apoptosis in metastatic cancers as 90% of metastatic cancers have active their telomerase activated. The telomerase targeted suicide gene containing MSCs would also eliminate several risks associated with MSC self renewal and differentiation after administration as MSCs also have active telomerase and would eventually undergo apoptosis reducing the risk of generating secondary cancers.
Another possible failure of detection of BM-MSC homing to prostate cancer site which might be the insensitivity of the methods embraced. Authors also commented on the power of BEAMing PCR and inability to detect low levels of donor DNA in patient samples. Current approaches can detect MSCs which are ≥1% of the total tumor mass. It is possible that the BM-MSCs had homed to prostate cancer sites but this homing was lower than the detectable limit with the methods used. This issue can be tackled with optimized period for MSC administration to surgery relay which was 4–6 days to increase to the total number of MSCs homing to the prostate cancer sites. Although the initial cohort who had administration of MSCs 28 days prior to the surgery, the total number of MSCs administered yet to be less than optimal. Similarly, the total number of cells administered might be increased to increase the load of MSCs in the circulation to have higher percentage of MSCs in the prostate cancer sites.
Furthermore, Schweizer et al. 2019 selected patients with Gleason Score 6. These tumors are defined to be low grade and less aggressive (10) and possible have not activated angiogenic factors to have clear access to the circulation. Therefore the systemically administered MSCs cannot be recruited to the tumor site even if the necessary inflammation signal is present. The patients with more aggressive prostate cancer might have been selected to detect MSC homing to prostate cancer sites. It is also possible that allogenic MSC homing mechanism might only home to aggressive and metastatic prostate cancer sites and not to the primary prostatic cancer sites. Therefore autologous MSCs should also be considered with this setting given that the patients enrolled have already donated MSCs or are in suitable condition to donate MSCs.
Safety of systemically administered BM-MSCs in prostate cancers
Authors have indicated that patients enrolled in this study did not have any adverse effects due to systemic administrations of BM-MSCs and the adverse effects observed were attributed to the prostatectomy. The safety of the MSC administration was mainly assessed based on the influence on surgery and recovery after surgery. Although the clearance of systemically administered MSCs is within 24 hours both in animal models and humans (11), the differentiation of uncleared MSCs by the liver presents a significant risk in systemically administered MSCs. The patients in the study by Schweizer et al. 2019 were not reported have any problems with MSC administration in 30 days. However, the detectable levels of neoplastic transformation of unrelated tissues might take longer than 30 days. Therefore we suggest a follow up period of 5 years of the patients to rule systemically administered allogenic MSCs are safe for prostate cancer patients.
Cancer specific delivery vehicles: mesenchymal stem cells
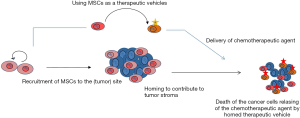
Systemically administered and genetically engineered BM-MSCs might have a higher potential in patients with prostate cancer. As previously suggested, active telomerase targeting of MSCs together with self suicide genes might increase the success rate of MSC based cancer therapeutics thus might minimize the potential risks associated with use of genetically modified MSCs (7). However, active telomerase is also substantial in sustained proliferation and immortalization of MSCs. Therefore, in order to establish a safe and successful delivery of chemotherapeutic drugs to primary prostate cancer sites using MSCs, prostate cancer specific cellular markers should also be considered when reprogramming the MSCs. One of the possible ways would be the recruitment of allogenic MSCs carrying therapeutic agent to the tumor site after the inflammation is enhanced. The autologous MSC homing contributes to the tumor stroma and subsequently induces the recruitment of allogenic MSCs which carry the therapeutic agent. MSCs as vehicles deliver and release the therapeutic agent into the tumor cell mass causing death in tumor cells (Figure 1). The combination of target genes and self suicide genes would maximize the homing potential of MSCs and minimize the risk of cancer development as a result of using engineered MSCs.
Conclusions
In conclusion, Schweizer et al. 2019 showed that allogenic BM-MSCs are not detectable in primary prostate cancers after systemically administered to patients. The possible approaches discussed here might prove to increase allogenic BM-MSC homing to the primary prostate cancer sites. Overall we share authors’ comments on why the BM-MSCs might not have homing to primary tumor side was not sufficient. However it is clear that the main principle behind this study was good although further improvements on targeting mechanism yet to be done. MSCs have substantial potential to be used as vehicles to deliver chemotherapeutic drugs to solid tumors provided that targeting the tumors is highly specific. Whether it is telomerase or tissue specific targeting, the main problem with cancer therapeutics is specific targeting and thus delivery of the chemotherapeutic agents to the primary tumor sites. Therefore, Schweizer et al. 2019 and clinical trials similar to their work are critical to enhance our understanding of possible mechanisms to overcome this problem.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Serakinci N, Christensen R, Fahrioglu U, et al. Mesenchymal stem cells as therapeutic delivery vehicles targeting tumor stroma. Cancer Biother Radiopharm 2011;26:767-73. [Crossref] [PubMed]
- Kortesidis A, Zannettino A, Isenmann S, et al. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood 2005;105:3793-801. [Crossref] [PubMed]
- Ries C, Egea V, Karow M, et al. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 2007;109:4055-63. [Crossref] [PubMed]
- Honoki K, Tsujiuchi T. Senescence bypass in mesenchymal stem cells: a potential pathogenesis and implications of pro-senescence therapy in sarcomas. Expert Rev Anticancer Ther 2013;13:983-96. [Crossref] [PubMed]
- Christensen R, Alsner J, Brandt Sorensen F, et al. Transformation of human mesenchymal stem cells in radiation carcinogenesis: long-term effect of ionizing radiation. Regen Med 2008;3:849-61. [Crossref] [PubMed]
- Kim JH, Lee HJ, Song YS. Stem cell based gene therapy in prostate cancer. Biomed Res Int 2014;2014:549136. [Crossref] [PubMed]
- Serakinci N, Cagsin H. Programming hMSCs into Potential Genetic Therapy in Cancer. Critical Reviews in Eukaryotic Gene Expression. [Crossref]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Fidler IJ, Kripke ML. The challenge of targeting metastasis. Cancer Metastasis Rev 2015;34:635-41. [Crossref] [PubMed]
- Knüchel R. Gleason Score 6 - Prostate Cancer or Benign Variant? Oncol Res Treat 2015;38:629-32. [Crossref] [PubMed]
- Singleton A, Khong D, Chin LY, et al. An engineered biomarker system to monitor and modulate immune clearance of cell therapies. Cytotherapy 2017;19:1537-45. [Crossref] [PubMed]


