
Testicular germ cell tumors: the changing role of the pathologist
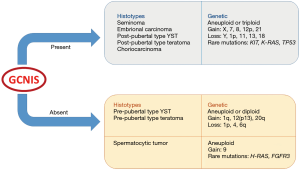
Testicular germ cell tumors (TGCTs) are a heterogeneous group of neoplasms derived from neoplastic transformation of germ cells in the testis, but with different epidemiological, biological and clinical settings, from early life to adulthood. In the latest years, several studies led to a deeper understanding of the genetic and biological events characterizing the development and progression of these neoplasms, at the basis of their divergent clinical behaviour. As a result, major and minor modifications regarding their classification criteria and staging parameters have been introduced. The recently published article by Lobo et al. highlighted the new challenges that TGCTs pose to pathologists (1). The latest WHO classification of TGCTs assigns a primary taxonomic role to germ cell neoplasia in situ (GCNIS), which is considered the main precursor lesion of TGCTs (2). In the past, different names have been used to refer to this entity, including “carcinoma in situ” (CIS), “intratubular germ cell neoplasia, unclassified” (IGCNU) and “testicular intraepithelial neoplasia” (TIN) (3). GCNIS is histologically defined as the neoplastic germ cells exclusively localized within the seminiferous tubules. These cells are characterized by enlarged hyperchromatic nuclei, clumped chromatin and often prominent nucleoli and closely resemble seminoma cells (4). In classic GCNIS, the neoplastic germ cells are distributed along the basal membrane of normal-sized seminiferous tubules, with still recognizable Sertoli cells but in absence of spermatogenesis. Involvement of seminiferous tubules is usually patchy and pagetoid spread of GCNIS into tubules with retained spermatogenesis and into the rete testis may also be seen (5,6). Apart from the so far described classical form, two other specific types of GCNIS are recognized, namely intratubular seminomas and intratubular non-seminomas. These variants are almost constantly associated with the presence of classic GCNIS as well as an invasive germ cell tumor and are histologically characterized by a more evident architectural alteration of the tubules, which appear enlarged and completely filled with neoplastic cells with loss of the Sertoli component. Neoplastic cells in intratubular seminoma are morphologically indistinguishable from those of GCNIS (seminoma-like cells) while a higher degree of cellular pleomorphism with greater atypia is seen in intratubular non-seminomas, whose neoplastic cells show morphological and immunohistochemical similarity to embryonal carcinoma cells and are frequently associated with intratubular necrosis and calcifications. Intratubular yolk sac tumor-like cells and teratoma-like cells have been described only anecdotally (6,7). It has been argued that intratubular seminomas and non-seminomas could represent an intermediate step between the classic in situ neoplasia and invasive tumors. An alternative hypothesis is that these more advanced forms of GCNIS represent retrograde colonization of seminiferous tubules by invasive tumors (8). Based on the presence of GCNIS, in the latest WHO classification, TGCTs were divided into non-GCNIS-derived TGCTs (mainly pre-pubertal) and GCNIS-derived TGCTs (mainly post-pubertal). The former includes prepubertal-type teratoma, prepubertal-type yolk sac tumor, mixed teratoma and yolk sac tumor prepubertal-type and spermatocytic tumor. The latter include seminoma, embryonal carcinoma, post-pubertal-type yolk sac tumor, choriocarcinoma, post-pubertal-type teratoma and mixed germ cell tumors (2). The distinction of TGCTs in GCNIS-related forms and GCNIS-unrelated forms reflects underlying pathogenetic differences (7,9). In fact, GCNIS-related TGCTs are associated with aneuploidy or triploidy, gain of chromosomes X, 7, 8, 12p, 21 and loss of chromosomes Y, 1p, 11, 13, 18; rarely, mutation of KIT, K-RAS and TP53 are found. On the other hand, GCNIS-unrelated TGCTs show diploidy or aneuploidy with gain of chromosomes 1q, 12, 20q and loss of chromosomes 1p, 4 and 6p. In contrast with other GCNIS-unrelated TGCTs, genetic features of spermatocytic tumor include gain of chromosome 9 and rare mutations of H-RAS and FGFR3 (10) (Figure 1). Although GCNIS-related TGCTs typically occur in adolescents and adults, while GCNIS-unrelated TGCTs occur in children, it is now accepted that GCNIS-unrelated TGCTs may also be encountered in an older age group, with particular reference to the existence of prepubertal-type teratomas with adult onset (11). The recognition of prepubertal-type teratomas in postpubertal patients has important prognostic implications, as these neoplasms behave in a benign fashion similarly to the pre-pubertal cases with obvious consequences on clinical outcome and management of the patient. Lobo et al. in their Series strongly emphasize the need for adequate sampling of the tumor mass and adjacent parenchyma in order to correctly classify the neoplasm (1). It is easily understood that extensive sampling aimed at excluding the presence of GCNIS acquires a special relevance in the diagnosis of prepubertal-type teratomas in adult patients. As a consequence, the sample should be better entirely submitted to histopathological examination whenever achievable; otherwise, sampling should always be as generous as possible (1). The American Joint Committee for Cancer (AJCC) has recently revised the histological parameters determining the staging of TGCTs, as well (12) (Table 1). In particular, neoplastic infiltration of the epididymis and hilar soft tissue has been assigned a formerly unrecognized role in the pathological stage definition of TGCTs. Indeed, unlike the previous TNM classification (13), the infiltration of the epididymis or hilar soft tissue now defines the neoplasm as stage pT2 regardless of the presence of lymphovascular invasion (12). As a consequence, the extensive microscopic examination of the testicular hilar soft tissue has become mandatory (14,15). Currently, the College of American Pathologists (CAP) and the International Society for Urologic Pathology recommend routine sampling and reporting of hilar neoplastic extension (16). Yilmaz et al. published a paper where hilar soft tissue invasion was described as a significant prognostic factor in multivariate analysis (17). Hilar soft tissue is now addressed as the most common site of extra-testicular neoplastic extension in TGCTs. Yilmaz et al. evaluated the prognostic significance of several clinical and pathological features in a series of 148 non-seminomatous TGCTs and demonstrated that rete testis invasion was a significant prognostic factor in multivariate analysis (17). Interestingly, the recognized prognostic role of neoplastic hilar extension could explain the prognostic significance of direct neoplastic invasion of rete testis. In fact, rete testis could represent a mandatory anatomical passage that precedes the neoplastic spread into the hilum. It is still a matter of debate whether the neoplastic invasion of the rete testis plays an important role in the prognosis. Indeed, it is generally recommended to include rete testis invasion in the pathological report, but the AJCC considers rete testis invasion does not change the pathologic stage pT1. Although there is no convincing evidence to upstage TGCTs with rete testis invasion, it is well-known that rete testis invasion correlates with higher rates of metastases in seminomas and recurrences in all TGCTs (18,19). Furthermore, also Yilmaz et al. observed in their series an association between the invasion of rete testis and the presence of metastatic disease (17), and Lobo et al. have demonstrated an association between rete testis invasion and lymphovascular invasion (1).

Full table
Another newly introduced and quite debated change in TNM classification of TGCTs is the subclassification of pT1 pure seminomas based on tumor size, with a 3 cm cut-off. Such subcategorization of pT1a and pT1b for seminomas limited to the testis (including the rete testis) in absence of lymphovascular invasion has been adopted by the AJCC, but not introduced by the UICC, and its prognostic significance is still controversial. Unlike non-seminomatous TGCTs, which are biologically more aggressive and prone to early dissemination, tumor size has long been considered a predictor of relapse and a useful element for risk assessment in pure seminomas only. However, it had not previously been recognized the dignity of a staging parameter.
In their cohort, Lobo et al. found seminomas size >3 cm to be associated with presence of rete testis invasion and extensive necrosis (1). The hypothesis of the authors that larger tumor size in seminomas may represent a kind of predictor of extratesticular extension seems to find confirmation in another recently published study. The analysis of a large series of TCGTs showed a correlation between seminoma size >3 cm and metastatic status at presentation, thus supporting the AJCC introduction of pT1a/pT1b substaging (20). Undoubtedly, further investigation is needed to shed light on the significance of tumor size in GCTs, seminomatous and non-seminomatous, and it’s supposed clinical impact.
In the past decades, some Authors proposed the presence of worrisome morphological features in seminomas, including cytological atypia, necrosis, high mitotic count and a larger tumor size, to correlate with a poorer clinical outcome. As a result, for a long time, pure seminomas characterized by a more marked nuclear pleomorphism, with larger nuclei and increased mitotic activity, have been labelled as “anaplastic” (21). However, considering the existing evidence in support of this distinction still weak and inconclusive, and the criteria adopted to define anaplasia unclear and poorly reproducible, the anaplastic variant has been removed from WHO 2016 edition and the use of such a definition is no longer recommended (2). In line with the new trend, Lobo at al could not describe a significant association between stage and the presence of atypical features in seminomas (1). It has been argued that the development of atypical morphological features, which can also be limited to a circumscribed area of the whole tumor mass, could herald a transition from pure seminomas to more aggressive tumor type, that should also detectable by a switch in their immunohistochemical profile with acquired expression of CD30 and cytokeratin 8/18. Once more, published data in this setting are insufficient and show disagreement (22,23). Further research is invoked to confirm or refute this theory but, most importantly, the importance of extensive sampling of the specimen in order to circumvent the problem of tumor heterogeneity strongly emerges. Apart from the already mentioned morphological and immunohistochemical features, some biomarkers like serum levels of beta-HCG have been investigated during time but all failed to give good results (24). Meanwhile, the early identification of those seminomas with a more aggressive behavior and with a tendency to distant metastases and recurrence still remains an open issue.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Lobo J, Costa AL, Vilela-Salgueiro B, et al. Testicular germ cell tumors: revisiting a series in light of the new WHO classification and AJCC staging systems, focusing on challenges for pathologists. Hum Pathol 2018;82:113-24. [Crossref] [PubMed]
- Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs - Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016;70:93-105. [Crossref] [PubMed]
- Berney DM, Looijenga LH, Idrees M, et al. Germ cell neoplasia in situ (GCNIS): evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 2016;69:7-10. [Crossref] [PubMed]
- Emerson RE, Cheng L. Premalignancy of the testis and paratestis. Pathology 2013;45:264-72. [Crossref] [PubMed]
- Berney DM, Lee A, Shamash J, et al. The association between intratubular seminoma and invasive germ cell tumors. Hum Pathol 2006;37:458-61. [Crossref] [PubMed]
- Lau SK, Weiss LM, Chu PG. Association of intratubular seminoma and intratubular embryonal carcinoma with invasive testicular germ cell tumors. Am J Surg Pathol 2007;31:1045-9. [Crossref] [PubMed]
- Oosterhuis JW, Kersemaekers AM, Jacobsen GK, et al. Morphology of testicular parenchyma adjacent to germ cell tumours. An interim report. APMIS 2003;111:32-40. [Crossref] [PubMed]
- Berney DM, Lee A, Randle SJ, et al. The frequency of intratubular embryonal carcinoma: implications for the pathogenesis of germ cell tumours. Histopathology 2004;45:155-61. [Crossref] [PubMed]
- Sperger JM, Chen X, Draper JS, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA 2003;100:13350-5. [Crossref] [PubMed]
- Giannoulatou E, Maher GJ, Ding Z, et al. Whole-genome sequencing of spermatocytic tumors provides insights into the mutational processes operating in the male germline. PLoS One 2017;12:e0178169. [Crossref] [PubMed]
- David S, András F, Endre K, et al. More Cases of Benign Testicular Teratomas are Detected in Adults than in Children. A Clinicopathological Study of 543 Testicular Germ Cell Tumor Cases. Pathol Oncol Res 2017;23:513-7. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual, 8th Edition. 2017.
- Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual, 7th Edition. 2009.
- Berney DM, Algaba F, Amin M, et al. Handling and reporting of orchidectomy specimens with testicular cancer: areas of consensus and variation among 25 experts and 225 European pathologists. Histopathology 2015;67:313-24. [Crossref] [PubMed]
- Dry SM, Renshaw AA. Extratesticular extension of germ cell tumors preferentially occurs at the hilum. Am J Clin Pathol 1999;111:534-8. [Crossref] [PubMed]
- Tickoo SK, Reuter VE, Amin MB, et al. Protocol for the Examination of Specimens from Patients with Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis. Available online: http://webapps.cap.org/apps/docs/committees/cancer/cancer_protocols/2012/Testis_12protocol_3200.pdf
- Yilmaz A, Cheng T, Zhang J, et al. Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod Pathol 2013;26:579-86. [Crossref] [PubMed]
- Bonet AS, Muñoz-Delgado EG, Vico FJ, et al. Analysis of clinical-pathologic variables, staging and prognostic groups, and therapeutic results of 106 germ-cell testicular tumors. Arch Esp Urol 2011;64:972-80. [PubMed]
- Valdevenito JP, Gallegos I, Fernández C, et al. Correlation between primary tumor pathologic features and presence of clinical metastasis at diagnosis of testicular seminoma. Urology 2007;70:777-80. [Crossref] [PubMed]
- Farooq A, Jorda M, Whittington E, et al. Rete Testis Invasion Is Consistent With Pathologic Stage T1 in Germ Cell Tumors. Am J Clin Pathol 2019;151:479-85. [Crossref] [PubMed]
- Johnson DE, Gomez JJ, Ayala AG. Anaplastic seminoma. J Urol 1975;114:80-2. [Crossref] [PubMed]
- Gallegos I, Valdevenito JP, Miranda R, et al. Immunohistochemistry expression of P53, Ki67, CD30, and CD117 and presence of clinical metastasis at diagnosis of testicular seminoma. Appl Immunohistochem Mol Morphol 2011;19:147-52. [Crossref] [PubMed]
- Som A, Zhu R, Guo CC, et al. Recurrent seminomas: clinical features and biologic implications. Urol Oncol 2012;30:494-501. [Crossref] [PubMed]
- Milose JC, Filson CP, Weizer AZ, et al. Role of biochemical markers in testicular cancer: diagnosis, staging, and surveillance. Open Access J Urol 2011;4:1-8. [PubMed]


