Concurrent liposomal paclitaxel and cisplatin chemotherapy improved outcomes for locally advanced esophageal squamous cell carcinoma treated with intensity-modulated radiotherapy
Introduction
Esophageal cancer (EC) is one of the most common cancers worldwide. Each year, there are 480,000 new cases of EC and 400,000 patients who die of this disease (1). Surgical resection remains the main method of curing the disease; however, over two-thirds of patients with EC cannot receive surgical resection because of the presence of advanced disease or remote metastasis (2). Definite chemoradiotherapy (CRT) is an alternative treatment for patients who are not candidates for surgical resection. In the Radiation Therapy Oncology Group (RTOG) 85-01 trial, the 5-year survival rate was 26% in patients who received combined CRT compared with 0% in patients receiving radiotherapy alone (3).
Recently, new radiotherapy modality or novel regimens of chemotherapy have been investigated to improve local control and long-term survival (4-6). Intensity-modulated radiotherapy (IMRT), which has been employed in the clinic in recent years, uses computer-controlled linear accelerators to deliver precise radiation doses to the planning target volume (PTV) (6,7). Therefore, IMRT can increase the radiation dose to the tumor to improve the local control rate while sparing normal tissues, which provides advantages over conventional radiotherapy (8). Although IMRT combined concurrent paclitaxel plus cisplatin to treat EC has been previously reported on by Tu et al., it was a single-arm study (9). To the best of our knowledge, there has been no study comparing IMRT alone versus IMRT with concurrent paclitaxel plus cisplatin in treating locally advanced ESCC. From October 2011 to December 2013, 72 patients with locally advanced esophageal squamous cell carcinoma (ESCC) received IMRT or IMRT combined with chemotherapy in our institute. In this study, we aimed to determine whether chemotherapy consisting of liposomal paclitaxel and cisplatin improved the prognosis of locally advanced ESCC receiving intensity-modulated radiotherapy. Safety profiles were also compared between these two types of treatments.
Methods
Patients
Patients were included in this study if they had locally advanced ESCC. All of the patients had an upper endoscopic examination with tumor biopsy, barium esophagography, and chest and abdominal computed tomography (CT) scans to determine their clinical stages. Patients were staged based on the 6th edition of the American Joint Committee on Cancer (AJCC) staging system. Patients were excluded from this study if they had esophageal perforation or prior surgery. From October 2011 to December 2013, 72 patients with newly-diagnosed ESCC were retrospectively included in this study. The median age was 69 (range: 50–84), and 51 patients were male (70.8%). All the patients had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1. In the 72 patients, 36 patients received IMRT combined with chemotherapy, while 36 patients received IMRT alone.
Treatments
IMRT
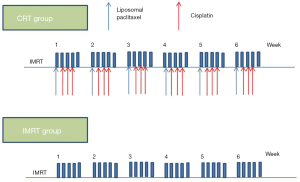
All patients received IMRT (Figure 1) (10). Generally, the gross target volume (GTV) consisted of the primary tumor and involved lymph nodes that were determined by contrast-enhanced CT. A margin of 0.5–0.8 cm was added in axial and 3 cm in a longitudinal direction to the primary tumor to define the clinical target volume (CTV). All the lymphatic drainage regions were radiated prophylactically according to the site of the primary tumor. Based on the CTV, a 0.3 cm margin was added in all directions to construct the PTV. The prescription dose to cover at least 95% of the PTV was 50 Gy. The dose for the primary tumor and involved lymph nodes was increased to 60–66 Gy. The radiation was delivered at 2.0 Gy per fraction and 5 fractions per week. The V20 for the lungs and the V30 for the heart was within 28% and 30%, respectively. The maximum tolerated dose for the spinal cord was 45 Gy.
Chemotherapy
For patients who received chemotherapy (1), the regimen was administered as follows (Figure 1): liposomal paclitaxel (35 mg/m2, d1) plus cisplatin (25 mg/m2, d2–d4) administered weekly, for 6 weeks.
Evaluation of response and follow-up
At one month after the completion of therapy, the response to IMRT or IMRT combined with chemotherapy was assessed using Response Evaluation Criteria in Solid Tumors (RECIST 1. 1) (11). Chemotherapy-associated adverse events were evaluated according to National Cancer Institute Common Toxicity Criteria Version 3.0 (12). Toxicities caused by radiation including radiation esophagitis and radiation pneumonitis were evaluated according to the toxicity criteria of RTOG and the European Organization for Research and Treatment of Cancer (EORTC) grading (13). Regular follow-up was carried out at least once three months to within 2 years after the completion of therapy and every 6 months thereafter. Routine follow-up included at least physical examination, endoscopy, or barium esophagography and contrast-enhanced CT.
Statistics
The Student’s t-test or Mann-Whitney U test was used to compare continuous variables between two groups. D’Agostino-Pearson omnibus normality test was used to examine if the values came from Gaussian distribution. Student’s t-test was used if the values passed normality test, or we used Mann–Whitney U-test. Categorical variables were compared by means of Fisher’s exact test. Overall survival (OS) was defined as the time from the initiation of therapy until death or last follow-up. Progression-free survival (PFS) was calculated from the time of therapy initiation to the time of local failure, metastasis, or last follow-up. Survival curves were plotted by Kaplan-Meier method, and the log-rank test was utilized for comparison, and variables consisting of gender, age, size of the tumor (T), lymph node involvement, and treatment were included in this analysis. Multivariate analysis was done by multivariate Cox model. All statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA), SPSS (version 19.0) software (IBM Corporation, Armonk, NY, USA). Statistical significance was defined as a P value less than 0.05.
Results
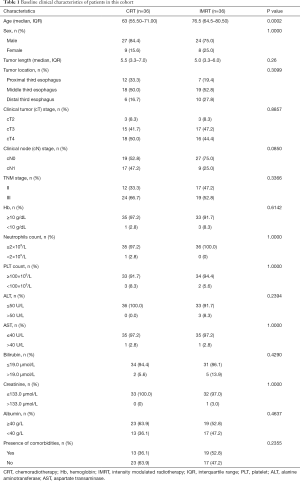
Baseline characteristics of the two arms are summarized in Table 1. The patients treated by IMRT plus chemotherapy were significantly younger than those treated by IMRT alone (median: 63 vs. 76.5, P=0.0002). There were no significant differences in other clinical characteristics between the two groups. However, there was a trend that the IMRT group was enriched for clinical N0 disease (P=0.0850).
Full table
Response
The response rates are summarized in Table 2. The overall response rate (ORR) for all the patients (n=72) was 73.6%. Thirty-six patients had a complete response (CR), and 17 patients had a partial response (PR). Thirty-one patients in the CRT group achieved CR or PR, while 22 patients in the IMRT alone group had a CR or PR. The ORR in the CRT group was significantly higher than that in the IMRT group (P=0.0309).

Full table
Follow-up
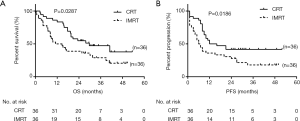
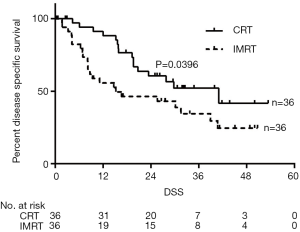
The last follow-up day was December 31st, 2015, and the median follow-up was 19.6 months [inter-quartile range (IQR), 8.3–31.8]. Of the 72 patients, 26 patients were still alive (17 of 36 patients in the IMRT plus chemotherapy group and 9 of 36 patients in the IMRT group). Median OS for patients was 29.7 months in the IMRT plus chemotherapy group and 12.9 months in the IMRT alone group [P=0.0287, hazards ratio (HR) 0.52 (95% CI, 0.29–0.93), Figure 2A]. OS was 83.3% [95% confidence interval (CI), 82.9–95.4%] at 1 year, 54.8% (95% CI, 54.3–71.2%) at 2 years, and 47.2% (95% CI, 46.7–64.4%) at 3 years in the IMRT plus chemotherapy group, compared with 50.0% (95% CI, 49.5–66.3%), 38.9% (95% CI, 38.4–54.8%), and 28.9% (95% CI, 28.4–44.4%) respectively, in the IMRT alone group. Median PFS was 14.0 months in the IMRT plus chemotherapy group and 6.5 months in the IMRT alone group [P=0.0186, HR 0.50 (95% CI, 0.28–0.89), Figure 2B]. PFS in the in the IMRT plus chemotherapy group was 55.6% (95% CI, 55.1–71.9%) at 1 year, 41.7% (95% CI, 41.2–57.8%) at 2 years, and 41.7% (95% CI, 41.2–57.8%) at 3 years, compared with 36.1% (95% CI, 35.6–51.8%), 27.8% (95% CI, 27.3–42.5%), and 18.0% (95% CI, 17.6–31.1%) respectively, in the IMRT alone group [HR 0.53 (95% CI, 0.28–0.87)]. Analysis of disease-specific survival (DSS) showed that patients in the IMRT plus chemotherapy group had a significantly higher DSS compared with patients in the IMRT group [median DSS: 41 vs. 15.2 months, P=0.0396, HR 0.52 (95% CI, 0.27–0.96)] (Figure 3).
Univariate analysis and multivariate Cox analysis of OS and PFS
Gender, age, clinical tumor stage (cT2/cT3 vs. cT4), clinical node stage (cN0 vs. cN1), and inclusion of chemotherapy were included in the univariate analysis. Only being female [P=0.036, HR 0.456 (95% CI, 0.219–0.949), Table 3] and inclusion of chemotherapy [P=0.032, HR 0.525 (95% CI, 0.291–0.946)] predicted better OS in our study. There was a trend that clinical lymph node involvement predicted worse survival [P=0.062, HR 1.777 (95% CI, 0.972–3.247)]. Multivariate Cox analysis revealed that being female and use of chemotherapy independently predicted a better OS [HR 0.439 (95% CI, 0.211–0.913), P=0.028; HR 0.505 (95%CI 0.280–0.913), P=0.024]. Regarding PFS, being female [P=0.035, HR 0.471 (95% CI, 0.234–0.948)] and inclusion of chemotherapy [P=0.025, HR 0.525 (95% CI, 0.298–0.923)] predicted better PFS. In multivariate Cox analysis, being female [P=0.036, HR 0.475 (95% CI, 0.236–0.954)] and use of chemotherapy [P=0.027, HR 0.528 (95% CI, 0.300–0.929)] were independently associated with superior PFS.

Full table
Subgroup analysis
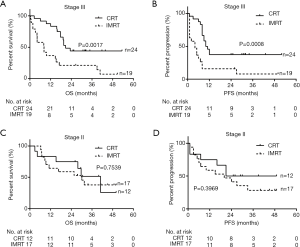
We explored the clinical impact of the addition of chemotherapy in patients with stage II and stage III diseases. In patients with stage III disease, we found that patients treated with CRT had a significantly superior OS (20.7 vs. 8.2 months, respectively, P=0.0017, Figure 4A) and PFS (10.5 vs. 4 months, respectively, P=0.0008, Figure 4B) as compared to patients receiving IMRT alone. However, in patients with stage II disease, CRT did not show a significant advantage over IMRT, in terms of OS (40.9 vs. 30.1 months, respectively, P=0.7539, Figure 4C) and PFS (35 vs. 20 months, respectively, P=0.3969, Figure 4D).
Safety
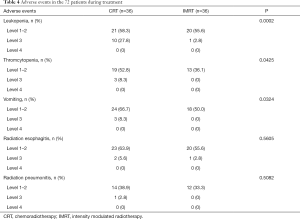
Adverse events for each arm are summarized in Table 4. The most common nonhematologic toxicities included grade 1 to 2 nausea, radiation esophagitis, and radiation pneumonitis. The incidence of grade 1 to 2 nausea was significantly higher in the IMRT plus chemotherapy group than in the IMRT alone group. The incidence of radiation esophagitis or radiation pneumonitis was similar between the two groups. Among hematologic toxicities, grade 3 to 4 neutropenia and thrombocytopenia were experienced by 11 patients and 3 patients, respectively. Grade 3 to 4 neutropenia and thrombocytopenia were more common in the IMRT plus chemotherapy group (P=0.0002, P=0.0425, Table 4). Also, grade 3 to 4 vomiting was more common in the IMRT plus chemotherapy group (P=0.0324, Table 4).
Full table
Discussion
In the present study, we discovered that IMRT combined with chemotherapy significantly improved both the OS and PFS of patients with locally advanced ESSC as compared to IMRT alone. Multivariate Cox analysis confirmed that the inclusion of chemotherapy as an independent factor is predictive of a favorable prognosis.
For patients with unresectable tumors or contraindications to surgery, radiation constitutes the backbone of treatment, which is of curative potential. With the advent of specialized CT scanners and planning software, IMRT has the ability to delineate tumors and organs accurately, thus allowing for optimal dose delivery to the tumor target volume and adjacent normal tissues (14). Furthermore, the development of effective drugs with fewer toxicities have made it possible to improve the outcome of patients with EC using concurrent CRT. Paclitaxel is a drug isolated from the bark of Taxus brevifolia, which interferes with the breakdown of microtubules during cell division (15). Preclinical and clinical data from studies in other cancers showed significant additive or synergistic activity for paclitaxel/cisplatin combination chemotherapy (16). Infusional paclitaxel has shown promising efficacy in the treatment of advanced esophageal squamous cell cancer (17). Weekly chemotherapy with paclitaxel and cisplatin and concurrent radiotherapy has been used as preoperative treatment in locally advanced EC, achieving a 3-year survival rate of 35% (18).
For patients with local but unresectable EC, CRT remains the only treatment of potentially curative intent. In the prospective randomized clinical trial, RTOG 85-01, combined chemotherapy, significantly prolonged OS compared with radiation alone (3). This study established the role of CRT in patients with localized advanced EC who opt for non-surgical treatment (3). In the JCOG 0604 study, CRT with concurrent S-1 and cisplatin showed acceptable toxicity and favorable 3-year survival (61.9%), which further affirmed the role of concurrent CRT in the treatment of localized advanced EC (19).
However, until now, there has been no standard regimen for CRT of EC. The meta-analysis by Pöttgen et al. reported a 2-year OS rate between 35% and 58% of patients treated by definite CRT (20). The 2-year OS rate of IMRT plus chemotherapy group in our study was within this range. In our study, the chemotherapy regimen was composed of liposomal paclitaxel and cisplatin. The 3-year OS rate of IMRT plus chemotherapy group was higher than that of the study by Shim et al. (21), which used weekly docetaxel and cisplatin as chemotherapy. Although the chemotherapy regimen was similar to that of the IMRT plus chemotherapy group in our study, the patients in the study by Shim et al. were all in stage III and IV. The advanced stage in their study accounted for the inferior 3-year OS of patients treated by concurrent CRT. In the study by Hsieh et al., 39 patients received IMRT plus chemotherapy, which consisted of intravenous cisplatin (20 mg/m2) for 1 hour and continuous intravenous infusion of fluorouracil (5-FU; 800 mg/m2) for 24 hours from day 1 to day 4 on week 1 and week 5 during radiotherapy (8). The 3-year OS was 28%, which was much lower than that of the patients treated by CRT in our study. Different regimens and intensities of chemotherapy in the two studies might have contributed to this difference; furthermore, the percentage of patients with clinical lymph node involvement (30/39, 76.9%) was much higher than that in our study.
Our study demonstrated that IMRT plus chemotherapy improved the outcome of patients with localized EC, compared with IMRT alone. Although the superiority of chemoradiotherapy to radiotherapy has already been proven in EC, the current study confirms this conclusion in the setting of IMRT. Despite the fact that there was a selection bias that younger patients were treated by concurrent CRT more frequently, survival analysis revealed that age was not a prognostic factor in our study. Multivariate Cox analysis demonstrated that inclusion of chemotherapy was an independent prognostic factor in predicting OS and PFS. In subgroup analysis, we found that IMRT plus chemotherapy significantly improved the outcome of patients with stage III disease, and this benefit was not observed in patients with stage II disease. This difference might be due to the fact that patients with stage II disease had a relatively favorable outcome (median OS: 30.1 month) with IMRT treatment; as a result, there was limited room for improvement in this subgroup of patients. In our cohort, being female independently predicted better OS and PFS; this might be due to the fact that females had a higher frequency of stage II disease compared with males (57.1% vs. 33.3%, respectively, P=0.0708), although without statistical significance.
The toxicities were acceptable in patients treated with concurrent CRT. Only 27.8% (10/36) of patients experienced grade 3 hematologic toxicities, and no grade 4 hematologic toxicities were observed. In another Chinese study using concurrent CRT contained paclitaxel plus cisplatin, 12.2% patients experienced grade 4 leukopenia; however, the patients in that study were much older than the patients in our study (22). Only 2 patients and 1 patient in the IMRT plus chemotherapy experienced grade 3 esophagitis and radiation pneumonitis, respectively. All these findings suggest that this combination was well-tolerated in our cohort.
The current study has several limitations. This is a retrospective study, so we were unable to balance the baseline clinical characteristics between the two arms. The patients in the IMRT plus chemotherapy group were much younger than those in the IMRT alone group, which made the direct comparison of the prognosis between the two groups questionable. Moreover, this is a single-center study with a relatively small size, so there exists bias impacting the conclusion due to the small sampling, especially in the subgroup analysis. Overall, it remains to be determined whether chemoradiotherapy using liposomal paclitaxel and cisplatin is superior to chemoradiotherapy using the standard chemotherapy regimen.
In summary, our study indicated that chemotherapy significantly improved the outcome of patients treated with IMRT. Large prospective studies are warranted to validate this conclusion in the future.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors declare no conflicts of interest.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This is a retrospective study, and the study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2018-SRFA-098).
References
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Thallinger CM, Raderer M, Hejna M. Esophageal cancer: a critical evaluation of systemic second-line therapy. J Clin Oncol 2011;29:4709-14. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Sasaki Y, Kato K. Chemoradiotherapy for esophageal squamous cell cancer. Jpn J Clin Oncol 2016;46:805-10. [Crossref] [PubMed]
- Tang HR, Ma HF, An SM, et al. A Phase II Study of Concurrent Chemoradiotherapy With Paclitaxel and Cisplatin for Inoperable Esophageal Squamous Cell Carcinoma. Am J Clin Oncol 2016;39:350-4. [Crossref] [PubMed]
- Lee SJ, Kim S, Kim M, et al. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: a randomized phase II study. BMC Cancer 2015;15:693. [Crossref] [PubMed]
- Gong Y, Wang S, Zhou L, et al. Dosimetric comparison using different multileaf collimeters in intensity-modulated radiotherapy for upper thoracic esophageal cancer. Radiat Oncol 2010;5:65. [Crossref] [PubMed]
- Hsieh HY, Yeh HL, Hsu CP, et al. Feasibility of intensity-modulated radiotherapy for esophageal cancer in definite chemoradiotherapy. J Chin Med Assoc 2016;79:375-81. [Crossref] [PubMed]
- Tu L, Sun L, Xu Y, et al. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiat Oncol 2013;8:75. [Crossref] [PubMed]
- Ge X, Yang X, Lu X, et al. Long-term clinical outcome of intensity-modulated radiation therapy for locally advanced esophageal squamous cell carcinoma. Tumori 2015;101:168-73. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Zhang W, Liu X, Xiao Z, et al. Efficacy of intensity-modulated radiotherapy for resected thoracic esophageal squamous cell carcinoma. Thorac Cancer 2015;6:597-604. [Crossref] [PubMed]
- Shi X, Sun X. Regulation of paclitaxel activity by microtubule-associated proteins in cancer chemotherapy. Cancer Chemother Pharmacol 2017;80:909-17. [Crossref] [PubMed]
- Kelsen D, Ginsberg R, Bains M, et al. A phase II trial of paclitaxel and cisplatin in patients with locally advanced metastatic esophageal cancer: a preliminary report. Semin Oncol 1997;24:S19-77-S19-81.
- Gu M, Li SY, Huang XE, et al. A phase II study on continuous infusional paclitaxel and 5-Fu as first-line chemotherapy for patients with advanced esophageal cancer. Asian Pac J Cancer Prev 2012;13:5587-91. [Crossref] [PubMed]
- Orditura M, Galizia G, Napolitano V, et al. Weekly chemotherapy with cisplatin and paclitaxel and concurrent radiation therapy as preoperative treatment in locally advanced esophageal cancer: a phase II study. Cancer Invest 2010;28:820-7. [Crossref] [PubMed]
- Tahara M, Fuse N, Mizusawa J, et al. Phase I/II trial of chemoradiotherapy with concurrent S-1 and cisplatin for clinical stage II/III esophageal carcinoma (JCOG 0604). Cancer Sci 2015;106:1414-20. [Crossref] [PubMed]
- Pöttgen C, Stuschke M. Radiotherapy versus surgery within multimodality protocols for esophageal cancer--a meta-analysis of the randomized trials. Cancer Treat Rev 2012;38:599-604. [Crossref] [PubMed]
- Shim HJ, Kim DE, Hwang JE, et al. A phase II study of concurrent chemoradiotherapy with weekly docetaxel and cisplatin in advanced oesophageal cancer. Cancer Chemother Pharmacol 2012;70:683-90. [Crossref] [PubMed]
- Song T, Zhang X, Fang M, et al. Concurrent chemoradiotherapy using paclitaxel plus cisplatin in the treatment of elderly patients with esophageal cancer. Onco Targets Ther 2015;8:3087-94. [PubMed]