
Catalpol may improve axonal growth via regulating miR-124 regulated PI3K/AKT/mTOR pathway in neurons after ischemia
Introduction
MicroRNAs (miRNAs), a class of endogenous 22-nucleotide noncoding RNAs, can inhibit gene expression by imperfectly pairing with the nucleotide sequences in the 3′-untranslated region of the target genes (1). It has been shown that miRNAs are able to regulate the expression of at least one third of the human genome. Aberrant miRNA expression may result in a wide range of diseases, including cancers, inflammation, developmental disorders (2) and stroke (3).
Mammalian genetic studies have confirmed the pivotal role of miRNAs in the regulation of gene expression (4). Among them, microRNA-124 (miR-124) is a brain-specific miRNA, which is highly conserved and expressed in neurons throughout the brain (5-7).
It was reported that miR-124 functions in not only the neuronal differentiation but also the neurite outgrowth and synaptogenesis (8). miR-124 seems to promote neurite outgrowth in mouse P19 cells by regulating members of the RhoGTPase family (9). In primary cortical neurons, ectopic expression of miR-124 enhances neurite outgrowth. Over-expression of miR-124 in the developing Drosophila sensory neurons suppresses dendritic branching (10). Interestingly, recent studies reveal that miR-124 is able to stimulate neurite outgrowth (9) and miR-124 has a negative regulation of PI3K and AKT. However, the mechanism by which miR-124 regulates axonal growth remains unclear.
Catalpol, an effective component of Rehmannia glutinosa Libosch, promoted neuron axon extension (11-13) and infarcted-brain angiogenesis via increasing VEGF, EPO, BDNF and GAP-43 expression in normal rat and stroke rat brain (13-15). However, it remains unclear how catalpol promote axon extension, here whether catalpol could regulate miR-124 to further promote axonal growth via PI3K/AKT/mTOR pathway in a rat stroke model was investigated. This study may provide a new target for the treatment of cerebral ischemia.
Methods
Animals
The whole study protocol was approved by the Ethics Committee of Chongqing Medical University and all the procedures were done according to the Chinese Guidelines for the Care and Use of Laboratory Animals. Sprague-Dawley rats (female and male rats, 200–250 g) were purchased from the Experimental Animal Center, Chongqing Medicine University (Chongqing, China, SCXK2012-0006) and housed under controlled environment (22±2 °C, 12 h light/dark cycle, free access to food and water) in the Experimental Animal Center, College of Pharmaceutical Sciences, Southwest University (Chongqing, China). Female and male rats were made a pair, and 24 h neonatal rats were chosen and sacrificed for isolating the primary neurons.
Materials
Catalpol (purity >98%) was purchased from Liu Bo Bai Niao Biological Technology Co., Ltd. (Shi Jiazhuang, China). DMEM was purchased from Hyclone (Logan, Utah, USA). Neurobasal, B27, fetal bovine serum (FBS), L-glutamine, and DMEM sugar-free medium were purchased from GIBCO (Gaithersburg, MD, USA). PBS was purchased from Dingguo Biological Company (Beijing, China). Pen-strep, and 0.25% Trypsin was purchased from Genview (Beijing, China). MTT, DMSO and L-polylysine were purchased from Sigma-Aliquot (St Louis, MO, USA). Except that the anti-p-S6 antibody was from Cell Signaling Technology (USA), all the antibodies used in this research were purchased from Wuhan Mitaka Company (Wuhan, China). Trizol was purchased from Ambion. Acetone and trichloromethane were purchased from Chongqing Science and Technology Company (Chongqing, China). Ethanol was from Chongqing Pratt & Whitney Limited Company (Chongqing, China). Isopropyl alcohol was purchased from Chengdu Kelon Chemical Reagent Factory (Chengdu, China). DEPC water was from Sangon Biotech (Shanghai, China). microRNA124 antagomir, microRNA124 agomir and ribo FECT nd Consumablesn/reper transfection kit were purchased from Guangzhou Rui Bo Biotechnology Company (Guangzhou, China). miRNA reverse transcription kit was purchased from Tiangen Biochemical Company (Beijing, China) and TaKaRA. SYBR Green qPCR kit was purchased from Vazyme Biotech (Nanjing, China). Protein antibodies and primers were purchased from Abcam Company, Cell Signaling Technology, Proteintech Company and Sangon Biotech Company, respectively.
Cell culture and transfection
The primary neurons were cultured as previously described (16) with some modifications. Briefly, the cerebral cortex from rat no more than 7 d after birth was collected and cut into pieces as small as possible. Then, the cortical tissues were incubated with 0.25% trypsin at 37 °C for 10 min for the preparation of single cell suspension. Then, cells were cultured in DMEM medium containing 10% FBS for 8 h, and subsequently the medium was refreshed with FBS-free Neurobasal medium containing 2% B27. Cells which were grown for 7 d were used in following experiments.
The transfection steps were as follows (17,18): cells were seeded into 6-well plates containing 500 µL of medium at a density of 1×107 cells/well. After 24 h, cells were completely adherent and were transfected. In brief, 250 µL of ultrapure water was added to 5 nmol miRNA-124 antagomir/agomir; 50 µL of 1× ribo FECT™ CP buffer was used to dilute 2.5 µL of 20 µM miRNA stock solution, and incubated at room temperature for 5 min. 5 µL of 1× ribo FECT™ CP Reagent, gently mix, followed by incubation at room temperature for 10 min. The ribo FECT™ CP mixture was added to 442.5 µL of cell culture medium (final concentration of miRNA reagent: 100 nM). The cells were cultured at 37 °C, in a CO2 incubator for 6 h, and then cells were collected for following experiment.
Immunophenotyping
For the immunophenotyping of primary neurons, MAP-2 as a marker was detected. In addition, the purity of primary neurons was analyzed as follow: purity = number of DAPI+ and MAP-2+ cells/number of DAPI+ cells (19).
Establishment of oxygen-glucose deprivation/reperfusion (OGD/R) model
Briefly, the prepared neurons were cultured in DMEM glucose-free medium and an environment (BINDER CB150, Germany) with 5% CO2 and 0.2% O2 at 37 °C for 2 h. Then, cells were subjected to re-oxygenization for 4 h. The successfully damaged neurons were of phenotype of contracted cells (19).
Grouping and treatments
Cells were divided into three groups: control group, miRNA124 agomir group, and miRNA124 antagomir group. To explore the mechanism, cells were divided into seven groups: control group, OGD group (OGD/R), miRNA124 agomir group, miRNA124 agomir plus catalpol group, miRNA124 antagomir group, miRNA124 antagomir plus catalpol group, and catalpol group. Before OGD/R, miRNA124 antagomir and microRNA124 agomir were transfected into neurons for 6 h by using ribo FECT nd Consumablesn/reper transfection kit.
MTT cell viability and LDH release assay
The cell survival rate was detected by MTT assay. The absorbance was detected with Biotek microplate reader (ELx800, USA) at 490 nm. The cell mortality was detected by LDH assay. The absorbance was detected at 450 nm. All the procedures were performed according to the manufacturer’s instructions.
Immunocytochemistry
For immunocytochemistry, cells were fixed in 4% paraformaldehyde at 4 °C for 20 min. After washing thrice (5 min for each) with 1× PBS, cells were blocked with 5% BSA at 37 °C for 1 h, and then incubated with primary antibody (MAP-2; 1:50) at 4 °C overnight. After washing thrice (5 min for each) with 1×PBS, cells were incubated with Alexa Fluor-coupled secondary antibody (1:100) for 1 h. Then, cells were mounted with ProLong® Gold reagent either with or without DAPI. Images were captured under a fluorescence microscope (DFC310, Leica, Germany) and then analyzed by IPP. Specifically, the average fluorescence density = total fluorescence density/number of cells.
Western blotting
Primary neurons were collected and lysed in RIPA buffer containing protease and phosphatase inhibitors (Dingguo, China) on ice for 5 min. After centrifugation (13,000 r/min, 4 °C), the resulting supernatant was collected as the cytoplasmic extract and the sediments were collected for nuclear extract. The protein concentrations were determined with BCA method. Then, 30 µg of proteins was loaded onto 8–10% SDS-polyacrylamide gels for separation. Proteins on the gels were then transferred onto PVDF membranes which were blocked with 3% BSA for 1 h to prevent non-specific binding. Thereafter, the membranes were incubated overnight at 4 °C with following primary antibodies: rabbit anti-p-S6 (1:100), rabbit anti-GAP-43, rabbit anti-PI3K, rabbit anti-mTOR, rabbit anti-p-mTOR, rabbit anti-PTEN, rabbit anti-p-TrkB, rabbit anti-TrkB (1:1,000), rabbit anti-BDNF (1:300). Following incubation, the membranes were washed, and proteins were visualized with chemiluminescence (ECL from GE Healthcare) after incubation with peroxidase-conjugated secondary antibodies (Proteintech; 1/1,000). Protein bands were captured with the Typhoon PhosphorImager (GE Healthcare), and the integrated density of each band was quantified with ImageJ software. β-Tubulin and GAPDH were used as loading controls (18).
RNA extraction and qRT-PCR
Total RNA was extracted from cultured neuronal cells with TRIzol at 6 h after transfection. Then, miRNA reverse transcription kit (Tiangen biochemical company, China) was used for reverse transcription of extracted RNA. qRT-PCR was performed in triplicates using SYBR Green on CFX96 Touch (Bio-Rad CFX 96, Hercules, CA). The relative expression of target genes was normalized to that of β-actin with 2−ΔΔCt method (18). The primers used in the study were listed in Tables 1 and 2.

Full table

Full table
Statistical analysis
All the experiments were repeated at least three times; all the images were analyzed with IPP software. Comparisons were done with one way analysis of variance (ANOVA), and statistical analysis was performed with SPSS version (20.0). Data are expressed as means ± standard deviation (SD). A value of P<0.05 was considered statistically significant.
Results
Fluorescence detection
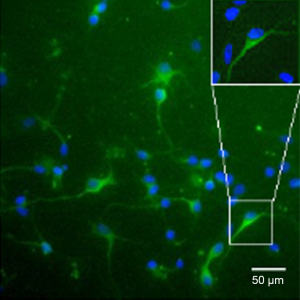
MAP-2 was a marker of neurons and mainly found in the cytoplasm and skeleton. By MAP-2 staining, the immunophenotype of neurons was confirmed, and the cellular purity was found to be higher than 96% (Figure 1).
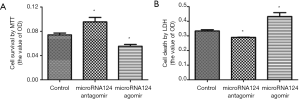
MiR-124 antagomir promotes cell survival and reduces cell death
MiRNA124 antagomir could promote neuronal cell survival and reduce cell death (P<0.05 vs. control); miRNA124 agomir could reduce neuronal cell survival and promote cell death (P<0.05 vs. control) (Figure 2A,B). These suggest miRNA124 is associated with the regulation of neuronal cell survival and death.
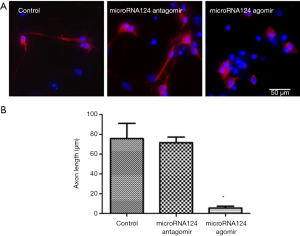
MiR-124 antagomir up-regulates GAP-43 expression to promote axonal growth
After treatment with miRNA124 antagomir, the number of MAP-2 positive cells increased significantly as compared to the control group (P<0.05), but the axonal length remained unchanged. After treatment with miRNA124 agomir, the number of MAP-2 positive cells was higher than in the control group (P<0.05), but the axonal length was significantly lower than in control group (P<0.05) (Figure 3A,B). These indicate miRNA124 may regulate axonal growth by regulating cell survival and axonal length. Increased axon length promotes contacting with surrounding cells, facilitating the transmission of information and nutrient delivery, which contribute to the survival of injured neuronal cells and newborn neurons.
MiR-124 antagomir promotes axonal growth via PI3K/AKT/mTOR/p-S6/GAP-43 pathway
MiRNA124 antagomir significantly increased the mRNA expression of PI3K, AKT and S6 (P<0.05 vs. control group); microRNA124 agomir dramatically reduced the mRNA expression of PI3K, AKT and S6 (P<0.05 vs. control group) (Figure 4A,B,C).
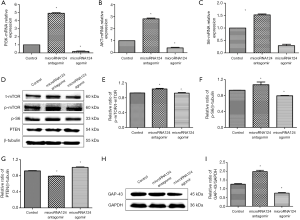
MiRNA124 antagomir up-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05 vs. control group); miRNA124 agomir down-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05 vs. control group) (Figure 4D,E,F). MiRNA124 antagomir down-regulated PTEN protein expression (P<0.05 vs. control group); miRNA124 agomir up-regulate PTEN protein expression (P<0.05 vs. control group) (Figure 4D,G). This suggests miRNA124 antagomir up-regulates protein expression of PTEN, a negative regulator of PI3K/AKT/mTOR pathway, to activate PI3K/AKT/mTOR pathway. MiRNA124 antagomir up-regulated the expression of GAP-43 to promote axonal growth (P<0.05 vs. control group); miRNA124 agomir down-regulated the expression of GAP-43 to compromise axonal growth (P<0.05 vs. control group) (Figure 4H,I). This suggests miRNA124 regulates PI3K/AKT/mTOR/p-S6/GAP-43 pathway and inhibiting miRNA124 may activate PI3K/AKT/mTOR pathway.
Catalpol reduces OGD/R-induced up-regulation of miR-124
The expression of miRNA124 significantly increased after OGD/R (P<0.05 vs. control group), and catalpol reversed OGD/R-induced increase in miRNA124 expression (P<0.05 vs. control group) (Figure 5).

Catalpol ameliorates OGD/R-induced loss of cortical neuronal cells via miR-124
After OGD/R, cell survival decreased and cell death increased significantly (P<0.05 vs. control group); addition of miRNA124 agomir markedly reduced cell survival and promoted cell death (P<0.05 vs. control group); concomitant treatment with miRNA124 agomir and catalpol significantly promoted cell survival and reduced cell death (P<0.05 vs. control group); addition of miRNA124 antagomir promoted cell survival and reduced cell death (P<0.05 vs. control group); in addition, concomitant treatment with miRNA124 antagomir and catalpol further significantly promoted cell survival and reduced cell death (P<0.05 vs. control group) (Figure 6A,B). These suggest that catalpol may regulate OGD/R-treated neuronal survival and death via miRNA124.

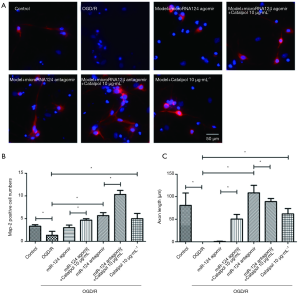
Catalpol up-regulates GAP-43 expression via miR-124 to promote axonal regeneration
After OGD/R, axons was impaired, MAP-2 positive cells decreased, and axonal length reduced significantly (P<0.05); after addition of microRNA124 agomir, MAP-2 positive cells and axonal length were comparable to those in OGD/R group; concomitant treatment with miRNA124 agomir and catalpol significantly promoted axonal regeneration, up-regulated MAP-2 positive cells and increased axonal length (P<0.05); addition of miRNA124 antagomir markedly promoted axonal regeneration, up-regulated MAP-2 positive cells and increased axonal length (P<0.05); meanwhile, concomitant treatment with miRNA124 antagomir and catalpol further significantly promoted axonal regeneration and up-regulated MAP-2 positive cells (P<0.05); treatment with catalpol dramatically promoted axonal regeneration, up-regulated MAP-2 positive cells and increased axonal length (P<0.05) (Figure 7A,B,C). These suggest catalpol may regulate cell survival and axonal length to promote axon growth via miRNA124.
Catalpol attenuates OGD/R induced damage to cortical neurons via miR-124 mediated regulation of PI3K/AKT/mTOR pathway
After OGD/R, the mRNA expression of PI3K, AKT and S6 decreased significantly (P<0.05 vs. control group); addition of microRNA124 agomir markedly down-regulated the mRNA expression of PI3K and S6 (P<0.05 vs. control group), but had no effect on AKT mRNA expression; concomitant treatment with miRNA124 agomir and catalpol dramatically upregulated the mRNA expression of AKT and S6 (P<0.05 vs. control group); addition of miRNA124 antagomir significantly increased the mRNA expression of PI3K, AKT and S6 (P<0.05); meanwhile, concomitant treatment with miRNA124 antagomir and catalpol further significantly elevated the mRNA expression of PI3K, AKT and S6 (P<0.05 vs. control group); catalpol significantly increased the mRNA expression of PI3K, AKT and S6 (P<0.05 vs. control group) (Figure 8A,B,C).
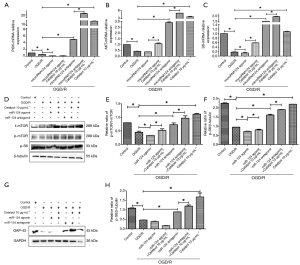
After OGD/R, p-mTOR/t-mTOR and p-S6 protein expression decreased significantly (P<0.05 vs. control group); addition of miRNA124 agomir markedly down-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05); concomitant treatment with miRNA124 agomir and catalpol dramatically up-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05); addition of miRNA124 antagomir significantly up-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05 vs. control group); meanwhile, concomitant treatment with miRNA124 antagomir and catalpol further significantly up-regulated p-mTOR/t-mTOR and p-S6 protein expression (P<0.05 vs. control group); treatment with catalpol markedly increased p-mTOR/t-mTOR and p-S6 protein expression (P<0.05 vs. control group) (Figure 8D,E,F). These suggest catalpol may regulate PI3K/AKT/mTOR pathway to protect neurons against OGD/R via miRNA124.
After OGD/R, GAP-43 protein expression decreased significantly (P<0.05 vs. control group); addition of miRNA124 antagomir significantly up-regulated GAP-43 protein expression (P<0.05 vs. control group); in addition, concomitant treatment with miRNA124 antagomir and catalpol further significantly up-regulated GAP-43 protein expression (P<0.05 vs. control group) (Figure 8G,H). These suggest catalpol may up-regulate GAP-43 protein expression via miRNA124.
Discussion
In cells, a variety of signaling pathways interact with each other to form the complex network, which is responsible for the positive and negative regulations of cell microenvironment. PI3K/AKT/mTOR signaling pathway has been found to be involved in the regulation of cell metabolism, growth, proliferation and survival. After PI3K activation, it can phosphorylate downstream proteins AKT and p-AKT to activate mTOR, ultimately regulating cell survival, proliferation, differentiation and migration (20). In the present study, results showed PI3K and AKT expression decreased after OGD/R, which was accompanied by reduced cell viability and axonal injury. These were similar to those reported in an animal model of cerebral infarction that up-regulation of p-AKT protected neurons by improving cell survival and reducing apoptosis (21).
MiRNA-124 is brain-specific and has a higher expression in the brain (22,23). It has been reported that the cortex has the highest expression of miRNA-124 in the brain (24). In stroke disorders, miRNA-124 is associated with neuronal protection. After cerebral ischemia, the miRNA-124 expression increased, and the elevated miRNA-124 then negatively regulates PI3K and AKT expression, compromising neuronal survival. AKT has a variety of downstream targets, and the specific mechanisms are unclear. mTOR pathway mainly regulates cell survival, protein transcription and axonal growth. In this study, the role of PI3K/AKT/mTOR pathway in the axonal growth and cell viability was further investigated.
In order to elucidate the relationship between miRNA-124 and PI3K/AKT/mTOR pathway, miRNA-124 antagomir and agomir were used to inhibit and over-express miRNA-124, respectively, in neurons. Results showed that inhibition of miRNA-124 expression significantly activated this pathway and increased cell survival and axonal growth, suggesting that miRNA-124 has a negative regulation of PI3K/AKT/mTOR pathway. This may be explained as follows. First, inhibition of miRNA-124 promotes cell survival and increases neurons. Second, inhibition miRNA-124 up-regulates GAP-43 expression to promote axonal growth. Third, inhibition of miRNA-124 up-regulates BDNF expression, which provides nutrition for the neuron growth. Finally, inhibition of miRNA-124 activates PI3K/AKT/mTOR pathway to promote protein synthesis and transcription.
The expression of many miRNA is altered after cerebral ischemia (25). It was reported that, in stroke disorders, miRNA-124 was associated with neuronal protection (26). Results of this study showed that miRNA-124 expression increased after OGD/R, but catalpol ameliorated it. This may be explained as follows. First, catalpol up-regulates BDNF expression to provide nutrition for neuronal growth. Second, catalpol activates PI3K/AKT/mTOR pathway to promote BDNF expression as well as other protein synthesis and transcription, cell survival and axonal growth. Finally, catalpol up-regulates GAP-43 expression to promote axonal growth.
Interestingly, results of this study showed that miRNA-124 antagomir and catalpol had a synergistic effect on promoting neuronal survival and axonal growth. On the one hand, miRNA-124 antagomir inhibited miRNA-124 expression, thereby activating PI3K/AKT/mTOR pathway; moreover, catalpol down-regulated miRNA-124 expression and activated this pathway. Concomitant treatment with miRNA-124 agomir and catalpol reversed the inhibition of miRNA-124 agonists on neuronal survival and axonal growth.
In conclusion, results indicate that catalpol may down-regulate miRNA-124 to activate PI3K/AKT/mTOR pathway to improve cell viability, axonal growth, and neuronal survival after OGD/R, which provides a new target for the treatment of cerebral ischemia and other relevant diseases. To function in cells, does catalpol need any prior activation? It is possible that catalpol is targeted to a target, which requires further in-depth studies, such as epigenetic, which may be a promoter or region that is initiated first, and then initiates subsequent activation reactions.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (81873034), the Natural Science Foundation Project of CQ CSTC (cstc2014jcyjA10083 & cstc2018jcyjAX0158).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The whole study protocol was approved by the Ethics Committee of Chongqing Medical University and all the procedures were done according to the Chinese Guidelines for the Care and Use of Laboratory Animals. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chen CZ, Li L, Lodish HF, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004;303:83-6. [Crossref] [PubMed]
- Lee S, Vasudevan S. Post-transcriptional stimulation of gene expression by microRNAs. Adv Exp Med Biol 2013;768:97-126. [Crossref] [PubMed]
- Yang X, Tang X, Sun P, et al. MicroRNA-15a/16-1 Antagomir Ameliorates Ischemic Brain Injury in Experimental Stroke. Stroke 2017;48:1941-7. [Crossref] [PubMed]
- Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol 2013;425:3582-600. [Crossref] [PubMed]
- Forero DA, van der Ven K, Callaerts P, et al. miRNA genes and the brain: implications for psychiatric disorders. Hum Mutat 2010;31:1195-204. [Crossref] [PubMed]
- Gao FB. Context-dependent functions of specific microRNAs in neuronal development. Neural Dev 2010;5:25. [Crossref] [PubMed]
- Liang Y, Ridzon D, Wong L, et al. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 2007;8:166. [Crossref] [PubMed]
- Schratt G. Fine-tuning neural gene expression with microRNAs. Curr Opin Neurobiol 2009;19:213-9. [Crossref] [PubMed]
- Yu JY, Chung KH, Deo M, et al. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 2008;314:2618-33. [Crossref] [PubMed]
- Xu XL, Li Y, Wang F, et al. The steady-state level of the nervous-system-specific microRNA-124a is regulated by dFMR1 in Drosophila. J Neurosci 2008;28:11883-9. [Crossref] [PubMed]
- Wan D, Zhu HF, Luo Y, et al. Catalpol promotes axon growth and synaptogenesis in peri-infarct cortex in rats. Chin Pharmacol Bullet 2013;29:931-6.
- Wan D, Zhu HF, Luo Y, et al. Study of catalpol promoting axonal growth for cultured cortical neurons from rats. Zhongguo Zhong Yao Za Zhi 2007;32:1771-4. [PubMed]
- Zhu HF, Wan D, Luo Y, et al. Catalpol up-regulated GAP-43 protein expression and improved behavior outcome of focal cerebral ischemia rats. Chin Pharmacol Bullet 2007;23:1231-6.
- Dong W, Xian Y, Yuan W, et al. Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats' stroke model. J Ethnopharmacol 2016;191:169-79. [Crossref] [PubMed]
- Wan D, Xue L, Zhu H, et al. Catalpol Induces Neuroprotection and Prevents Memory Dysfunction through the Cholinergic System and BDNF. Evid Based Complement Alternat Med 2013;2013:134852. [Crossref] [PubMed]
- Xu SY, Hu YF, Li WP, et al. Intermittent hypothermia is neuroprotective in an in vitro model of ischemic stroke. Int J Biol Sci 2014;10:873-81. [Crossref] [PubMed]
- Gu X, Li A, Liu S, et al. MicroRNA124 Regulated Neurite Elongation by Targeting OSBP. Mol Neurobiol 2016;53:6388-96. [Crossref] [PubMed]
- Xu Z, Zhang K, Wang Q, et al. MicroRNA124 improves functional recovery and suppresses Baxdependent apoptosis in rats following spinal cord injury. Mol Med Rep 2019;19:2551-60. [PubMed]
- Xue Q, Liu Y, He R, et al. Lyophilized Powder of Catalpol and Puerarin Protects Neurovascular Unit from Stroke. Int J Biol Sci 2016;12:367-80. [Crossref] [PubMed]
- Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 2002;4:648-57. [Crossref] [PubMed]
- Noshita N, Lewen A, Sugawara T, et al. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 2001;21:1442-50. [Crossref] [PubMed]
- Bak M, Silahtaroglu A, Moller M, et al. MicroRNA expression in the adult mouse central nervous system. Rna 2008;14:432-44. [Crossref] [PubMed]
- Sempere LF, Freemantle S, Pitha-Rowe I, et al. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 2004;5:R13. [Crossref] [PubMed]
- Mishima T, Mizuguchi Y, Kawahigashi Y, et al. RT-PCR-based analysis of microRNA (miR-1 and -124) expression in mouse CNS. Brain Res 2007;1131:37-43. [Crossref] [PubMed]
- Zhang Y, Guo J. MicroRNA and cerebral ischemia. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2012;34:418-21. [PubMed]
- Zhu F, Liu JL, Li JP, et al. MicroRNA-124 (miR-124) regulates Ku70 expression and is correlated with neuronal death induced by ischemia/reperfusion. J Mol Neurosci 2014;52:148-55. [Crossref] [PubMed]


