
Primary colorectal lymphoma: computed tomography and double-contrast barium enema examination findings with histopathological correlation in 19 patients
Introduction
Primary colorectal lymphoma (PCL) is a rare disorder that has been reported to occur in 0.4% of all colorectal malignancies (1). The colon and rectum are also uncommon sites for lymphoma compared with the rest of the gastrointestinal tract, accounting for 10–20% of all cases of gastrointestinal lymphoma (2-6). The diagnostic criteria of PCL were initially established by Dawson et al. (7) in 1961. A diagnosis of PCL requires histological confirmation of a lymphoma fulfilling the following strict criteria: no superficial enlarged lymph nodes on first examination of the patient; chest radiographs showing no obvious enlargements of the mediastinal nodes; total and differential white blood cell counts within normal limits; laparotomy showing only regional nodes affected by disease; and tumor-free liver and spleen. Subsequently, the criteria were extended in the modern era to encompass a few wider ranges of manifestations, including all patients who present with lymphoma that apparently originated from a colorectal site (5,8,9), in accord with the alternative definition of extranodal lymphoma (10,11).
The imaging features of PCL are rarely described. Double-contrast barium enema (DCBE) and computed tomography (CT) are the two main radiographic procedures used to diagnose and evaluate colon lymphoma. In this article, we describe the spectrum of PCL via a retrospective evaluation of the imaging features and histopathological findings in 19 patients with pathologically proven PCL.
Methods
This study was approved by the Institutional Review Board at Cancer Hospital, Chinese Academy of Medical Sciences. Because the study was retrospective and the data were analyzed anonymously, the need for written informed consent was waived.
Patients
Patients who presented with predominant colorectal lesions were defined as having PCL according to the definition for primary gastrointestinal tract lymphoma proposed in previous reports (5,8,9). A search of the pathology files of colorectal neoplasms (n=17,108) at our institution revealed that PCL was diagnosed in nineteen patients with imaging (DCBE or CT) between January 2005 and December 2018. All these patients received histopathologic diagnosis of lymphoma and subtypes were classified according to immunohistochemical staining. Twelve patients were male, and seven were female (age range, 17–75 years). Sixteen patents underwent surgical resection; the other three patients only underwent colonoscopic biopsy. All 19 patients had undergone CT examination; seventeen patients underwent enhanced CT examination, and two patients underwent nonenhanced CT examination. Six patients also had undergone DCBE examination.
Image acquisition and review
The CT equipment and scan parameters were as follows: GE lightspeed 64-VCT: 120 kV, 450 mAs, thickness 5 mm, spacing 5 mm, reconstruction thickness 1.25 mm, reconstruction spacing 0.8 mm; GE lightspeed Ultra 8-MDCT: 120 kV, 450 mAs, thickness 5 mm, spacing 5 mm, reconstruction thickness 1.25 mm, reconstruction spacing 0.8 mm; Toshiba Aquilion 64-MDCT: 120 kV, 380 mAs, thickness 5 mm, spacing 5 mm, reconstruction thickness 1 mm, spacing 0.8 mm. Enhanced CT examinations commenced following a 65-s delay after intravenous injection with 85 mL of contrast medium (300 mg/mL) using a power injector at a rate of 2.5 mL/s. DCEB images were obtained using the following equipment: a Toshiba Mec ADR-100A digital gastrointestinal apparatus; and a Shimadzu DAR-3000 digital gastrointestinal apparatus.
The CT and DCBE findings were retrospectively reviewed in consensus by two radiologists who were aware of the diagnosis. The CT findings included the location and extent of the lesion, morphological patterns, presence of necrosis, characteristics of enhancement, dissemination of pericolonic fat, presence of regional lymph node enlargement and another organ involvement. The evaluation of the DCBE findings included the presence of a niche, filling defect, mucosal destruction, luminal narrowing, peristalsis, and ileocecal deformity. The large intestinal lymphoma pattern was classified into four types: a circumferential infiltrative form, an ulcerinfiltrative form, a focal/localized polypoid form, or a mesenteric nodal form (12-15). These findings were correlated with those of a colonoscopy and an examination of gross resected specimens.
Results
Clinical and pathological features of PCL
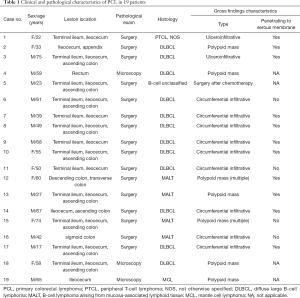
Clinical presentations included abdominal pain and discomfort in nine patients, bloody stools in five, and altered bowel habits in four; PCL was detected in one patient without any clinical symptoms by a health examination. According to the Ann Arbor staging scheme (16,17)), seven patients had disease confined to the colon (stage IE, 36.8%), eleven had regional lymph node involvement (stage IIE, 57.9%), and one had adjacent organ and tissue involvement (stage IVE, 5.3%).
Pathologically, non-Hodgkin lymphoma (NHL) was present in all patients. The histological details are presented in Table 1. Immunohistochemical staining revealed diffuse large B-cell lymphoma (DLBCL) in 12 patients (63.2%), B-cell lymphoma arising from mucosa-associated lymphoid tissue (MALT) in 4 patients (21.1%), unclassified B-cell lymphoma in 1 patient (5.3%), peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) in 1 patient (5.3%), and mantle cell lymphoma (MCL) in 1 patient (5.3%).
Full table
Imaging features of PCL
Forty-four colorectal segments were involved; sixteen lesions were located in the ileocecum, thirteen in the terminal ileum, ten in the ascending colon, one in the rectum, one in the descending colon, one in the transverse colon, one in the sigmoid colon and one in the appendix.
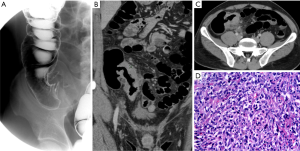
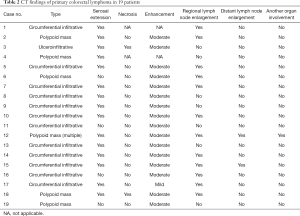
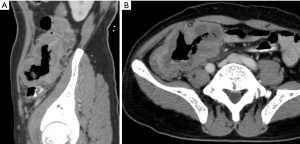
The CT findings indicated a circumferential infiltrative form presenting wall thickening (n=12) (Figures 1,2), a focal polypoid mass form (n=6) (Figure 3), and a cavitary/ulceroinfiltrative form (n=1) (Table 2, Figure 4). Two cases presented as aneurysmal dilatation (Figure 4). Of seventeen patients who underwent enhanced CT, two lesions exhibited central low attenuation with necrosis, while the other fifteen lesions manifested as homogeneous soft-tissue masses with mild or moderate enhancement (similar to the psoas muscle within the same slice). Fifteen lesions extended into the serous membrane or pericolonic fat, characterized by stranding or linear soft-tissue density in the pericolonic fat area. Regional lymphadenopathy, including bowel wall lymph nodes, pericolorectal lymph nodes, and mesentery root lymph nodes, was found in twelve patients; one case showed enclosure of the mesenteric vessels without invasion (Figure 3). Direct involvement of both the ovaries and diaphragm was evident in one patient. Intussusception of the terminal ileum was seen in one patient.

Full table

Six patients underwent DCBE examination (Table 3). A filling defect was found in all patients, a flat niche in four (Figure 2), mild mucosal destruction in three, luminal narrowing in all patients with slight luminal narrowing in three, and ileocecal deformation in three (Figure 1). Peristalsis was preserved in five patients.

Full table
Comparison of imaging and pathological findings
Among these 19 patients, the preoperative CT diagnoses were colon carcinoma in ten patients, lymphoma in five patients and undefined in four patients. Among the six patients who underwent DCBE, the preoperative diagnosis was lymphoma in two and colon carcinoma in four. The preoperative diagnostic accuracies of PCL were 26.3% (5/19) with CT and 33.3% (2/6) with DCBE.
Colonoscopy (n=3) or colectomy specimens (n=16) were available for all 19 patients. However, one patient received preoperative chemotherapy, and the pathological changes were obscure. According to the gross findings (n=18), two lesions manifested as ulcerative masses, seven as focal masses, and nine as diffuse thickness of the colorectal wall. The accuracy of CT classification was 83.3% (15/18). Both ulcerative lesions exhibited giant ulcerations with an irregular, elevated bottom; one lesion was located in the ileocecum, and the other involved the ileocecum and part of the ascending colon. CT demonstrated only one ulcerative lesion. According to surgical pathological findings (n=15), four lesions were limited to the superficial myometrium of the colorectal wall, while the other eleven lesions infiltrated the serous membrane and pericolorectal fat, which was 93.3% (14/15) consistent with the prospective CT diagnosis of whether the lesion infiltrated the serous layer.
Discussion
Clinical and pathological features of PCL
The incidence of PCL is greatest among those aged 50 to 70 years, with a mean age at presentation of between 50 and 55 years in Eastern Asian reports (18). In our study, the age range was wide (17–75 years old); five patients were younger than 40 years old. This finding may be correlated with a trend of tumors occurring in younger people. Our study shows a slight male predominance (male: female =12:7), which correlates well with the results of most previous studies (19-22). The most common presenting symptoms were abdominal pain and weight loss or changes in bowel habits, as previously reported in the literature (20-22). In our study, clinical symptoms included abdominal pain and discomfort in six patients, altered bowel habits in three patients, and bloody stools in three patients. None of the patients experienced weight loss, which may be correlated with most of our patients being diagnosed at an early stage (92.4% stage I or II). In turn, this phenomenon may be because of improved diagnostics, increased health awareness, or racial differences between Eastern and Western individuals.
In our study, NHL was present in all 19 patients. T-cell lymphoma was present in 1 (5.3%), and B-cell lymphoma was present in 18 (94.7%), including DLBCL in 12 patients (63.2%), MALT in 4 patients (21.1%), unclassified B-cell lymphoma in 1 patient (5.3%), and MCL in 1 patient (5.3%). DLBCL was the most common subtype of PCL, which correlates well with the results of most studies (20,23). MALT of the gastrointestinal tract most commonly affects the stomach, and colorectal involvement is uncommon. Such tumors are characterized by an indolent course with little evidence of dissemination outside the gut, but these tumors can transform into intermediate or high-grade lymphoma (24-26). In our series, the lesions of two MALT cases both infiltrated the serosal membrane and perienteric tissues; one of these patients manifested multiple lesions involving the transverse and descending colons and infiltrating the paracolic tissues and organs. T-cell lymphoma is usually very aggressive with a poor prognosis (27,28). Perforation of the bowel frequently occurs (29-32). In our series, T-cell lymphoma manifested pathologically as a diffuse lesion and deep ulceration.
Imaging features of PCL
PCL is likely to involve multiple segments of the colon. In our series, there was Forty-four colorectal segments were involved in 19 patients. According to previous studies in the literature, the most common location for colorectal lymphoma is the ileocecum, occurring in 30–60% of cases, probably because more lymphoid tissue is present in this region (2,22,33-35). Our study showed a higher rate, with 84.2% (16/19) of the lesions located in the ileocecum; among these, thirteen involved the terminal ileum. The ascending colon was the next most common site of occurrence (52.6%, 10/19) in our series. Some studies have shown that the rectum is another common site of PCL; however, there was only one (5.3%) case of rectum involvement in our study.
In our study, a circumferential lesion with colorectal wall thickening was the most common morphological pattern (68.4%, 13/19). These lesions presented as concentric wall thickening with a structure integrated with the intestinal wall on CT and circumferential narrowing of the colon with a smooth mucosal surface on DCBE. The smooth mucosal surface is caused by the submucosal infiltration of lymphoid cells rather than mucosal ulceration. Lymphoma does not elicit a desmoplastic response, and submucosal lymphoid infiltration weakens the muscularis propria of the bowel wall. Therefore, although PCL lesions are often long and circumferential, severe luminal narrowing is uncommon, and signs of aneurysmal dilatation can even occur in some cases, as in three cases in this study. Aneurysmal dilatation of the lumen is a characteristic sign and may be due to replacement of the muscularis propria and destruction of the autonomic nerve plexus by lymphoma (36).
Polypoid masses have been reported to be the most frequent form of PCL (1). However, focal polyploid masses were detected in 5 (26.3%) patients in this study; single, large polypoid masses on one side of the bowel wall were observed in three patients, while multiple focal polyploid masses in the colon were found in one patient. The differential diagnosis of this form of lymphoma is colon carcinoma or large colonic polyps. PCL manifests as larger masses than colon carcinoma. Additionally, PCL can extend beyond the colon wall and form a large peritoneal mass (1). In our series, focal polypoid masses were less common than circumferential lesions. The characteristic finding of this form is an eccentric mass.
Previously, it was believed that colon lymphoma has no or minimal mucosal ulceration. According to radiological studies in the literature (28), peripheral T-cell lymphoma of the colon manifests as either a diffuse or a focal segmental lesion and shows extensive mucosal ulceration on DCBE examination. This differentiation of this form from inflammatory bowel disease is difficult. In our study, the only case of T-cell origin lymphoma manifested as circumferential infiltration without ulceration on CT, while pathology revealed ulceration, which may have been because such pathological ulcerations are more difficulty to detect on CT images than on DCBE images.
According to a previous view, PCL with serosal or pericolonic fat extension was less common than colorectal carcinoma (36). However, in our study, there were eleven cases (73.3%, 11/15) pathologically proven and twelve cases (78.9%, 15/19) on CT with serosal extension or pericolonic fat extension. Therefore, serosal or pericolonic fat extension may not be a characteristic radiographic feature of colon carcinoma compared with PCL. The extension manifested as stranding densities in the pericolonic fat with an unclear serosal membrane or stripe-like opacities parallel to the bowel wall on CT. Compared with the appearance of colon carcinoma, these opacities were less acute and had fewer nodules on the bowel wall (37).
Twelve (63.2%) patients had regional lymph nodal involvement, which manifested as aggregated nodules or masses in the pericolonic fat space or lymph drainage area characterized by homogeneous soft-tissue lesions without necrosis, moderate enhancement and characteristic enclosure of the mesenteric vessels without invasion. When CT reveals the presence of an infiltrative process accompanied by enlarged lymph nodes in the abdomen or pelvis, lymphoma should be the primary consideration in the differential diagnosis and must be excluded by endoscopic biopsy.
Comparison of imaging and pathological findings
The diagnostic accuracy of PCL was 26.3% (5/19) with CT and improved to 33.3% (2/6) when combined with DCBE. Combining DCBE and CT examinations may offer improved diagnostic accuracy in difficult cases. In this study, all imaging misdiagnosed cases were diagnosed as colorectal cancer. The imaging features of PCL on DCBE included giant mucosal rugae, superficial submucosal filling defects, and changing lesion forms with compression on fluoroscopy compared to colorectal cancer. However, these imaging signs are usually subtle, non-specific and overlap with colorectal cancers. We suggested that this is the main cause for PCL imaging misdiagnosis, in addition to the very low prevalence of PCL. Although the CT diagnostic accuracy of PCL is even lower, the diagnostic ability of lesion morphological classification (83.3%) and serosal or pericolonic fat extension (93.3%) is satisfactory. As opposed to diagnosis, the value of CT is that it reveals the location and extent of the lesion and the involvement of adjacent organs and tissues as well as distant metastasis. Therefore, CT is very crucial for PCL staging and is currently performed as a routine exam in clinical practice.
Conclusions
PCL is a relatively rare malignant disease. The most common histological subtype of colorectal lymphoma is DLBCL. PCL usually presents circumferential or polyploid lesions in the ileocecum and cecum, with slight mucosal destruction and mild luminal narrowing on DCBE, along with moderate and homogeneous enhancement on CT. Aneurysmal dilatation of the lumen is sometimes observed. Although the imaging features of PCL overlap with those of other colon pathologies, such as carcinoma and inflammatory disease, CT is valuable in the evaluation of invasion extent and lymphadenopathy. Familiarity with the radiological features of PCL on DCBE and CT can help ensure correct diagnosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Lee HJ, Han JK, Kim TK, et al. Primary colorectal lymphoma: spectrum of imaging findings with pathologic correlation. Eur Radiol 2002;12:2242-9. [Crossref] [PubMed]
- Wong MT, Eu KW. Primary colorectal lymphomas. Colorectal Dis 2006;8:586-91. [Crossref] [PubMed]
- Green JA, Dawson AA, Jones PF, et al. The presentation of gastrointestinal lymphoma: study of a population. Br J Surg 1979;66:798-801. [Crossref] [PubMed]
- Contreary K, Nance FC, Becker WF. Primary lymphoma of the gastrointestinal tract. Ann Surg 1980;191:593-8. [Crossref] [PubMed]
- Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer 1978;42:693-707. [Crossref] [PubMed]
- Gay ND, Chen A, Okada CY. Colorectal Lymphoma: A Review. Clin Colon Rectal Surg 2018;31:309-16. [Crossref] [PubMed]
- Dawson IM, Cornes JS, Morson BC. Primary malignant lymphoid tumours of the intestinal tract. Report of 37 cases with a study of factors influencing prognosis. Br J Surg 1961;49:80-9. [Crossref] [PubMed]
- Herrmann R, Panahon AM, Barcos MP, et al. Gastrointestinal involvement in non-Hodgkin's lymphoma. Cancer 1980;46:215-22. [Crossref] [PubMed]
- Kim SJ, Choi CW, Mun YC, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer 2011;11:321. [Crossref] [PubMed]
- Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol 1997;8:727-37. [Crossref] [PubMed]
- Krol AD, le Cessie S, Snijder S, et al. Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol 2003;14:131-9. [Crossref] [PubMed]
- Megibow AJ, Balthazar EJ, Naidich DP, et al. Computed tomography of gastrointestinal lymphoma. AJR Am J Roentgenol 1983;141:541-7. [Crossref] [PubMed]
- Rubesin SE, Gilchrist AM, Bronner M, et al. Non-Hodgkin lymphoma of the small intestine. Radiographics 1990;10:985-98. [Crossref] [PubMed]
- Levine MS, Rubesin SE, Pantongrag-Brown L, et al. Non-Hodgkin's lymphoma of the gastrointestinal tract: radiographic findings. AJR Am J Roentgenol 1997;168:165-72. [Crossref] [PubMed]
- Byun JH, Ha HK, Kim AY, et al. CT findings in peripheral T-cell lymphoma involving the gastrointestinal tract. Radiology 2003;227:59-67. [Crossref] [PubMed]
- Rosenberg SA. Validity of the Ann Arbor staging classification for the non-Hodgkin's lymphomas. Cancer Treat Rep 1977;61:1023-7. [PubMed]
- Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989;7:1630-6. [Crossref] [PubMed]
- Jinnai D, Iwasa Z, Watanuki T. Malignant lymphoma of the large intestine--operative results in Japan. Jpn J Surg 1983;13:331-6. [Crossref] [PubMed]
- Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3861-73. [Crossref] [PubMed]
- Bairey O, Ruchlemer R, Shpilberg O. Non-Hodgkin's lymphomas of the colon. Isr Med Assoc J 2006;8:832-5. [PubMed]
- Stanojevic GZ, Stojanovic MP, Stojanovic MM, et al. Non-Hodgkin's lymphomas of the large bowel-clinical characteristics, prognostic factors and survival. Acta Chir Iugosl 2008;55:109-14. [Crossref] [PubMed]
- Fan CW, Changchien CR, Wang JY, et al. Primary colorectal lymphoma. Dis Colon Rectum 2000;43:1277-82. [Crossref] [PubMed]
- Cai S, Cannizzo F, Dunn KMB, et al. The role of surgical intervention in non-Hodgkin's lymphoma of the colon and rectum. Am J Surg 2007;193:409-12. [Crossref] [PubMed]
- Brown JA, Carson BW, Gascoyne RD, et al. Low grade gastric MALT Lymphoma: radiographic findings. Clin Radiol 2000;55:384-9. [Crossref] [PubMed]
- Yoo CC, Levine MS, Furth EE, et al. Gastric mucosa-associated lymphoid tissue lymphoma: radiographic findings in six patients. Radiology 1998;208:239-43. [Crossref] [PubMed]
- Park MS, Kim KW, Yu JS, et al. Radiographic findings of primary B-cell lymphoma of the stomach: low-grade versus high-grade malignancy in relation to the mucosa-associated lymphoid tissue concept. AJR Am J Roentgenol 2002;179:1297-304. [Crossref] [PubMed]
- The Non-Hodgkin's Lymphoma Classification Project. A clinical evaluation of the international lymphoma study group classification of non-hodgkin's lymphoma. Blood 1997;89:3909-18. [PubMed]
- Lee HJ, Han JK, Kim TK, et al. Peripheral T-cell lymphoma of the colon: double-contrast barium enema examination findings in six patients. Radiology 2001;218:751-6. [Crossref] [PubMed]
- Son HJ, Rhee PL, Kim JJ, et al. Primary T-cell lymphoma of the colon. Korean J Intern Med 1997;12:238-41. [Crossref] [PubMed]
- Hirakawa K, Fuchigami T, Nakamura S, et al. Primary gastrointestinal T-cell lymphoma resembling multiple lymphomatous polyposis. Gastroenterology 1996;111:778-82. [Crossref] [PubMed]
- Yamamoto K, Shiraishi T, Ajiki T, et al. A case of intestinal T-cell lymphoma with repeated episodes of perforation. Gastroenterol Jpn 1991;26:649-53. [Crossref] [PubMed]
- Kaneki T, Kawashima A, Akamatsu T, et al. Immunoblastic lymphadenopathy-like T-cell lymphoma complicated by multiple gastrointestinal involvement. J Gastroenterol 1999;34:253-9. [Crossref] [PubMed]
- Musallam KM, Hatoum HA, Barada K, et al. Primary colorectal lymphoma. Med Oncol 2010;27:249-54. [Crossref] [PubMed]
- Gonzalez QH, Heslin MJ, Davila-Cervantes A, et al. Primary colonic lymphoma. Am Surg 2008;74:214-6. [PubMed]
- Doolabh N, Anthony T, Simmang C, et al. Primary colonic lymphoma. J Surg Oncol 2000;74:257-62. [Crossref] [PubMed]
- Ghai S, Pattison J, Ghai S, et al. Primary gastrointestinal lymphoma: spectrum of imaging findings with pathologic correlation. Radiographics 2007;27:1371-88. [Crossref] [PubMed]
- Filippone A, Ambrosini R, Fuschi M, et al. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology 2004;231:83-90. [Crossref] [PubMed]


