
Increased neutrophils and IL-17A in a rare organizing pneumonia secondary to extrapulmonary operation
Introduction
Organizing pneumonia (OP), which used to be called bronchiolitis obliterans organizing pneumonia (BOOP), was first described as a clinical pathology syndrome characterized by granulation tissue in the lumens of small airways, alveolar ducts and the alveolar space by Epler et al. in 1985 (1). This condition is regarded as a common reaction of lung tissue to different injury factors and a mutual manifestation of a variety of diseases in the lungs rather than an independent disease. When the lung is injured by various acute and chronic factors, it can be manifested as diffuse alveolar damage, OP, acute fibrinous and OP and certain types of fibrotic lung disease (2) In 2001, the American Thoracic Society and the European Respiratory Society (ATS/ERS) referred to idiopathic OP as cryptogenic pneumonia (COP), which is recognized as a subtype of idiopathic interstitial pneumonia and OP with specific etiology as secondary organizing pneumonia (SOP) (3). As we know, a number of diseases and factors are correlated with OP, such as infection (4), connective tissue disease (CTD) (5,6), radiation therapy (7), drug reaction (8), cancer (9), hematological diseases (10), inflammatory bowel disease (11), organ transplantation (12), gastroesophageal reflux disease (13), toxic exposure (14), thyroid disease (15), and menstruation (16). In recent years, some cases of OP following chest surgery have also been reported (17). A case of acute fibrinous and OP after a surgical resection of rectal adenocarcinomas has recently been reported (18), which could still not prelude the effects of cancer. However, as far as we know, until now, no cases of OP secondary to nonthoracic surgery have been reported. Here, we report a case of OP secondary to internal fixation surgery for a left clavicular fracture.
Case presentation
A previously healthy 19-year-old patient was transferred to our hospital from the outer court with a 9-day history of fever, cough and expectoration in September, 2015. The patient experienced an internal fixation surgery for a left clavicular fracture 8 days before the outset of the symptoms. No obvious abnormalities were observed in the chest computer tomographic (CT) (Figure 1A) before the operation, and ceftizoxime was used to prevent infection after surgery. After the operation, the chest CT showed cluster and patchy shadows in the lower lobes of the bilateral lungs, but no pleural effusion was observed in either lung (Figure 1B). At first, the condition was considered a postoperative pulmonary infection, and a variety of antibiotics, including piperacillin-tazobactam, moxifloxacin, vancomycin, imipenem and linezolid, were successively given to the patient. Nevertheless, despite treatment with antibiotics and supportive drugs, the patient still continued to deteriorate (Figure 1C,D). Therefore, he was transferred to our hospital for further diagnosis and treatment. He did not have any chronic diseases except a history of chronic sickle cell anemia for more than 10 years. In addition, he did not have a history of smoking, alcohol consumption and specific medication or an exposure history to industrial toxicant, hazardous dust and radiation.
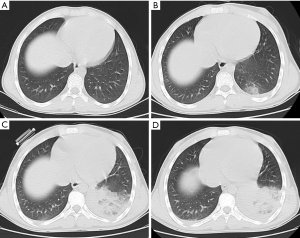
Physical examination revealed the patient had a temperature of 38.5 °C a heart rate of 115 beats/min, a respiratory rate of 20 breaths/min, a blood pressure of 120/80 mmHg and an oxygen saturation level of 95%. Lung examination showed tactile fremitus of the left lung was weakening, percussion of lower left lung was voiced, breathing of the left lower lung was low, breathing of the right lung was thick, and moist rale was heard in both of the lower pulmonary regions. Laboratory data suggested a white blood cell count of 10,720 cells·mm−3 (10.72 cells ×109 L-1) with 62.8% neutrophils, an erythrocyte sedimentation rate (ESR) of 93 mm/h and a C-reactive protein (CRP) level of 121 mg/L. Pleural effusion examination indicated extravasate. However, the etiological examination including bacteria, fungus and tubercule bacillus were all negative. In addition, the antinuclear antibody and antineutrophil cytoplasmic antibody counts were all negative, and the fiberoptic bronchoscopy examination showed no obvious abnormality.
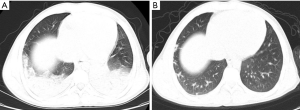
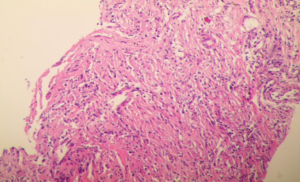
Initially, we considered the patient to be afflicted with hospital-acquired pneumonia. Therefore, we gave the patient a variety of broad-spectrum antibacterial agents, but the patient’s symptoms still did not improve after anti-infective treatment, and the lesion in chest CT was still progressing (Figure 2). Combined with the patient’s history, symptoms, auxiliary examinations and the response to drugs, we considered the possibility of OP. Therefore, a CT-guided percutaneous needle lung biopsy was performed on the patient, and the patient received an empirical therapy of 40 mg methylprednisolone per day according to our experience. The pathological findings showed interstitial fibrosis hyperplasia and a larger number of inflammatory cells infiltrated into lung interstitial tissue (Figure 3), which confirmed OP. His respiratory symptoms improved gradually, and his temperature gradually returned to normal after treatment with methylprednisolone for a week. A reexamination of CRP was 3.28 mg/L. He was discharged one day later and continued taking 20 mg prednisone acetate daily. After the patient was discharged from hospital, 36 days later, all symptoms disappeared, and a significant improvement in the chest CT was seen (Figure 2B). Therefore, the dose of prednisone acetate was reduced to 15 mg per day. Three months later, the dose of prednisone acetate was decreased to 10 mg for maintenance therapy. We have been following up with the patient, and no relapse was observed until now.
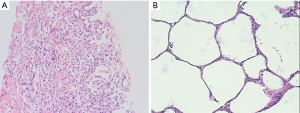
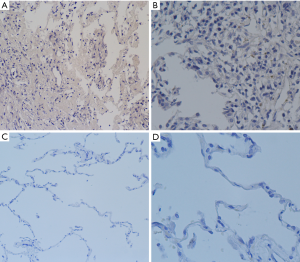
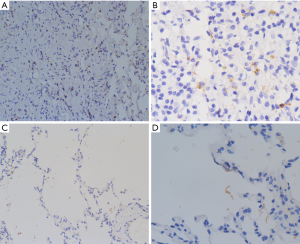
To further confirm the pathogenesis of OP secondary to extrapulmonary operation, we further analyzed hematoxylin and eosin (HE)-staining, alkaline phosphatase (ALP)-staining and immunohistochemical staining of pulmonary tissue slices of the patient compared with control subject after receiving the consent of the subjects. Our case also revealed that pulmonary inflammation was evident in the patient with organic pneumonia (Figure 4). Moreover, neutrophils in lung tissue of the patient were also increased (Figure 5). IL-17A is an important inflammatory cytokine that is closely associated with Th17 cell inflammation. To further verify that whether Th17 cells were involved in the development of OP, we performed an immunohistochemical staining analysis of IL-17A. Consequently, we found positive expression of IL-17A in the patient was increased (Figure 6). Consequently, we hypothesized that neutrophils and Th17 cells may be involved in the pathogenesis of OP.
Discussion
Many noninfectious processes, especially OP, are easily and mistakenly diagnosed as hospital acquired pneumonia. To date, the pathogenesis of OP is still not completely understood. Some scholars consider that alveolar epithelial cells and microvascular endothelial cells are damaged by multifarious factors, which start the repair mechanism of the body, promoting alveolar macrophages to produce abundant proinflammatory cytokines and chemokines, including interleukin 1 beta (IL-1β), IL-6, IL-8, tumor necrosis factor alpha (TNF-α) and CC chemokine ligand 18 (CCL18) (19). At the same time, the activation of macrophages and lymphocytes also enlarges the Th1 response, which further promotes the growth of granulation tissue and the infiltration of chronic inflammation cells in the pulmonary interstitium (20). Because of the injury of alveolar epithelial cells and microvascular endothelial cells, which damages the integrity of the alveolar wells, the newborn granulation tissue grows to the alveoli and alveolar ducts and other small airways, forming the OP. Recently, Shokri et al. revealed that an LPS-responsive beige-like anchor gene mutation may participate in the pathogenesis of OP (21). Bronchoalveolar lavage fluid (BALF) analysis of OP patients revealed a significant increase in the proportion of lymphocytes, neutrophils, eosinophils and mast cells and a significant decrease in the percentage of macrophages (22).
This report described one patient with OP attributed to internal fixation surgery for a left clavicular fracture. The patient had no symptoms, and the chest CT was normal before the operation, but the patient developed a fever, cough, expectoration and multiple patchy exudations in the chest CT postoperation. At first, we considered the diagnosis of hospital acquired pneumonia and gave the patient powerful anti-infective therapy, but no improvement was observed in clinical conditions and chest radiograph. Combined with patient's history, symptoms, laboratory examination and imagological examination, diagnosis and treatment process and response to drugs, we examined the possibility of a noninfectious disease, and CT-guided lung biopsy confirmed OP. Significant clinical and radiographic improvements were seen after initiation of corticosteroid therapy, which further confirmed the diagnosis of OP. In addition, no other causes of OP were observed in the patient. Therefore, the diagnosis of OP secondary to fracture internal fixation surgery was clear.
We have not found any reported cases of OP after fracture internal fixation surgery and other nonthoracic surgery. Therefore, this should be the first case report about an unusual OP secondary to extrapulmonary operation. In addition, this study suggests that neutrophils and IL-17A may be involved in the pathogenesis of OP. The case implied that extrapulmonary surgery may also be an important cause of OP. When a patient has a cough, fever and other symptoms after nonthoracic operation and repeated antibiotic treatment is ineffective, we should be cautious of the occurrence of OP.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China grant 81770031, Natural Science Foundation of Jiangsu Province BK20171501, the “Six Talent Peak” Project of Jiangsu Province 2013-WSN-059, a project from the Administration of Traditional Chinese Medicine of Jiangsu Province LZ13213 and a project from Jiangsu Provincial Commission of Health and Family Planning H201501. The funders had no roles in the in the design of the study, the collection, analysis, and interpretation of data or in writing the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The written informed consent was obtained from all subjects, and this study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University.
References
- Epler GR, Colby TV, McLoud TC, et al. Bronchiolitis obliterans organizing pneumonia. N Engl J Med 1985;312:152-8. [Crossref] [PubMed]
- Kligerman SJ, Franks TJ, Galvin JR. From the radiologic pathology archives: organization and fibrosis as a response to lung injury in diffuse alveolar damage, organizing pneumonia, and acute fibrinous and organizing pneumonia. Radiographics 2013;33:1951-75. [Crossref] [PubMed]
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [Crossref] [PubMed]
- Kim I, Lee JE, Kim KH, et al. Successful treatment of suspected organizing pneumonia in a patient with Middle East respiratory syndrome coronavirus infection: a case report. J Thorac Dis 2016;8:E1190-4. [Crossref] [PubMed]
- Okada H, Kurasawa K, Yamazaki R, et al. Clinical features of organizing pneumonia associated with rheumatoid arthritis. Mod Rheumatol 2016;26:863-8. [Crossref] [PubMed]
- Lee WS, Choi KH. Organizing pneumonia with atypical computed tomography findings in Sjogren's syndrome. Int J Rheum Dis 2015;18:482-4. [Crossref] [PubMed]
- Ochiai S, Nomoto Y, Yamashita Y, et al. Radiation-induced organizing pneumonia after stereotactic body radiotherapy for lung tumor. J Radiat Res 2015;56:904-11. [Crossref] [PubMed]
- Barjaktarevic IZ, Qadir N, Suri A, et al. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013;143:858-61. [Crossref] [PubMed]
- Eguchi T, Takasuna K, Fujiwara M, et al. Coexistence of a pulmonary adenocarcinoma with a focal organizing pneumonia. Interact Cardiovasc Thorac Surg 2011;13:444-6. [Crossref] [PubMed]
- Kimura Y, Imamura Y, Higaki K, et al. Case of polycythemia vera with unusual organizing pneumonia mimicking the clinical features of military tuberculosis and possibly caused by the involvement of neoplastic megakaryocytes. Pathol Int 2011;61:486-90. [Crossref] [PubMed]
- Dinneen HS, Samiullah S, Lenza C. Cryptogenic organizing pneumonia: a rare extra-intestinal manifestation of Crohn's disease. J Crohns Colitis 2014;8:177-8. [Crossref] [PubMed]
- Shino MY, Weigt SS, Li N, et al. Impact of Allograft Injury Time of Onset on the Development of Chronic Lung Allograft Dysfunction After Lung Transplantation. Am J Transplant 2017;17:1294-303. [Crossref] [PubMed]
- Liu JR, Xu XF, Zhou CJ, et al. Bronchiolitis obliterans organizing pneumonia due to gastroesophageal reflux. Pediatrics 2015;135:e1510-3. [Crossref] [PubMed]
- Yamanda S, Kobayashi S, Hanagama M, et al. Two Cases of Tsunami Dust Pneumonia: Organizing Pneumonia Caused by the Inhalation of Dried Tsunami Sludge after the 2011 Great East Japan Earthquake. Intern Med 2016;55:3645-53. [Crossref] [PubMed]
- Watanabe K, Senju S, Maeda F, et al. Four cases of bronchiolitis obliterans organizing pneumonia associated with thyroid disease. Respiration 2000;67:572-6. [Crossref] [PubMed]
- Yigla M, Ben-Itzhak O, Solomonov A, et al. Recurrent, self-limited, menstrual-associated bronchiolitis obliterans organizing pneumonia. Chest 2000;118:253-6. [Crossref] [PubMed]
- Guzman EJ, Smith AJ, Tietjen PA. Bronchiolitis obliterans-organizing pneumonia after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2000;119:382-3. [Crossref] [PubMed]
- Zhou CX, Tang TT, Huang LJ, et al. Methylprednisolone combined with low-dose indomethacin treating acute fibrinous and organizing pneumonia after a surgical resection of rectal adenocarcinoma: a case report and literature review. Eur Rev Med Pharmacol Sci 2016;20:2077-89. [PubMed]
- Cai M, Bonella F, Dai H, et al. Macrolides inhibit cytokine production by alveolar macrophages in bronchiolitis obliterans organizing pneumonia. Immunobiology 2013;218:930-7. [Crossref] [PubMed]
- Forlani S, Ratta L, Bulgheroni A, et al. Cytokine profile of broncho-alveolar lavage in BOOP and UIP. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:47-53. [PubMed]
- Shokri S, Nabavi M, Hirschmugl T, et al. LPS-Responsive Beige-Like Anchor Gene Mutation Associated With Possible Bronchiolitis Obliterans Organizing Pneumonia Associated With Hypogammaglobulinemia and Normal IgM Phenotype and Low Number of B Cells. Acta Med Iran 2016;54:620-3. [PubMed]
- Majori M, Poletti V, Curti A, et al. Bronchoalveolar lavage in bronchiolitis obliterans organizing pneumonia primed by radiation therapy to the breast. J Allergy Clin Immunol 2000;105:239-44. [Crossref] [PubMed]


