Preoperative D-dimer level is an independent prognostic factor for non-small cell lung cancer after surgical resection: a systematic review and meta-analysis
Introduction
Lung cancer is the most common malignant tumor and also the leading cause of cancer death worldwide (1). There are two major histological types of lung cancer, namely non-small cell lung cancer (NSCLC), which is reported to account for 85% of all lung cancers, and small cell lung cancer (SCLC), which accounts for only 15% of lung cancers (2). Currently, for resectable NSCLC, surgery still remains to be the preferred therapeutic option. Despite of advancement of surgical techniques, the 5-year survival rate for surgically treated NSCLC varied from 25% to 73% based on different disease stages (3). Moreover, even after curative surgical resection of NSCLC, the recurrence rate was reported to be as high as about 30% to 70% (4). Therefore, in order to better direct therapeutic and follow-up strategies for individualized therapy for each patient, it is of great value to explore potential prognostic factors for surgically treated NSCLC patients.
Despite of disease stage at diagnosis and performance status being the most important prognostic factors (5), D-dimer level has also recently been investigated as a potential prognostic factor of lung cancer patients (6-8). D-dimer is the fibrinolytic degradation products of crosslinked fibrin and is applied as a useful marker for the diagnosis of pulmonary embolism (9,10). It is reported that high pretreatment D-dimer level was observed in various malignant tumors and it was found to be an unfavorable prognostic factor for these malignant tumors including lung cancer (8). Even though several meta-analyses found the prognostic role of high D-dimer level in lung cancer, none of them conducted subgroup analysis for NSCLC, let alone for these surgically treated NSCLC, because no relevant studies focusing on surgically treated NSCLC specifically were available when these meta-analyses were conducted, and as a result, they all mixed NSCLC and SCLC together for analysis (6-8). However, NSCLC and SCLC were different pathologic types of lung cancers with different therapeutical strategies because of their distinct biology and genomic abnormalities (11) and even for NSCLC patients, surgical resection and non-surgical therapy yielded significantly different outcomes (12). As a result, it is reasonable that significant heterogeneities were observed in previous meta-analyses (6-8). Moreover, none of these previous meta-analyses focused on the outcome of disease-free survival (DFS) in lung cancer patients, which was an important parameter for evaluation of disease recurrence. Therefore, the value of preoperative D-dimer in predicting overall survival (OS) and DFS in patients with surgically treated NSCLC remains undetermined. In this study, we aimed to conducted a systematic review and meta-analysis to investigate the impact of high preoperative level of D-dimer on long-term survival of patients with surgically treated NSCLC. To our knowledge, this is the first meta-analysis specifically focusing on patients with surgically treated NSCLC.
Methods
Literature search
In order to retrieve relevant studies comprehensively, we systematically searched the following three website literature databases on January 28, 2019: PubMed, Embase, and Web of Science. We used the following search terms for search: “d-dimer” and “lung cancer”. We also comprehensively scanned all the references from the selected studies to further retrieve potential relevant studies.
Study inclusion and exclusion
Our study inclusion criteria were as follows: (I) either randomized controlled trials (RCTs) or observational studies compared survival of NSCLC patients with high preoperative D-dimer level with that of patients with normal preoperative D-dimer level; (II) all patient should be diagnosed with NSCLC and be surgically treated; (III) sufficient data of OS and DFS from multivariate analysis could be obtained for analysis; (IV) If studies were based on overlapping patients, the most completed one was chosen. We used the following criteria for study exclusion: (I) studies including patients with other types of lung cancers apart from NSCLC; (II) studies including NSCLC patients treated without surgical resection; (III) studies not published in English; (IV) conference abstracts, reviews, case reports, and experiment studies.
Data extraction and quality assessment
A standardized data collection form, which included first author, year of publication, study origin, disease stage, age, sample size, follow-up time, and study design, was applied for data extraction. Two authors (X Zheng and R Jiang) independently extracted and analyzed the outcome data by using the standardized data form. If there was a discrepancy between the two authors, the third author (HY Deng) would resolve it. The main outcomes for analysis consisted of hazard ratio (HR) of OS and DFS from multivariate analysis. The Jadad scale (13) would be applied to evaluate the quality of RCTs and the Newcastle-Ottawa Scale (NOS) as described previously (14), which consisted of three factors: patient selection, comparability of the study groups, and assessment of outcome, would be used to assess the quality and risk-of-bias of the observational studies. During the application of NOS, we would give out a score of 0–9 (allocated as stars) to each observational study. Here, the high-quality study was defined as one with a quality score of more than 6. The name of the first author and publishing year was used for identification in our meta-analysis.
Statistical analysis
We applied the STATA 12.0 package (StataCorp., College Station, TX, USA) to perform this meta-analysis based on the PRISMA guidelines (15) (Supplement file). We extracted HRs with 95% confidence interval (CI) directly from each original article and used them for comparing OS and DFS between patients with high preoperative level of D-dimer and these with normal preoperative level of D-dimer. The between-study heterogeneity was evaluated by the χ2-based Q statistics and I2 test, and a significant heterogeneity was defined as P<0.1 or I2>50%. When significant heterogeneity was observed, we would apply the random effects models for analysis. Otherwise, we would apply the fixed effects models. A sensitivity analysis was also conducted by sequential removal of each study. Here we applied a funnel plot as well as Begg’s test and Egger’s test (16) to assess publication bias. A two-sided P value of <0.05 was deemed as statistical significance.
Results
Description of the included studies
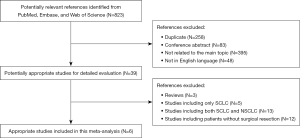
A flow chart for the process of study evaluation in our meta-analysis was shown in Figure 1. After systematic search, we retrieved a total of 823 papers. After initial assessment with titles and abstracts, we found 39 potential relevant papers for detailed evaluation with full text. Three review papers were excluded (6-8) and 30 papers were further excluded due to the fact that they included SCLC patients or NSCLC patients without surgical resection. Finally, only a total of 6 cohort studies with a total of 1,817 patients with surgically treated NSCLC were included for final analysis (17-22). One study (19) reported the results subgrouped by different cut-off values, and as a result, we extracted the data individually from its subgroup analysis for analysis. The main characteristics of these included studies were listed in Table 1. All these included patients had a stage I–III disease and were treated with surgical resection. The median age for all patients ranged from 60 to 69 years old. All studies except two had a relative long follow-up time. However, the cut-off value among these studies differed from each other. The main outcomes of OS and DFS were extracted from the multivariate analysis in each study, which could significantly avoid bias caused by confounding factors. We listed these main outcomes in Table 2, and four of these studies reported HRs for OS, while only three studies reported HRs for DFS.

Full table

Full table
Quality assessment and risk of bias
With only cohort studies included for analysis, we conducted the quality assessment and risk-of-bias analysis of these studies based on the NOS. Here we listed the quality assessment result of each study in Table 1. All these studies were evaluated as high quality, suggesting a very low risk of bias.
Meta-analysis of the impact of high preoperative D-dimer level on long-term survival of patients with surgically treated NSCLC
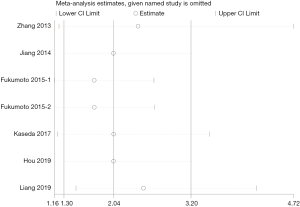
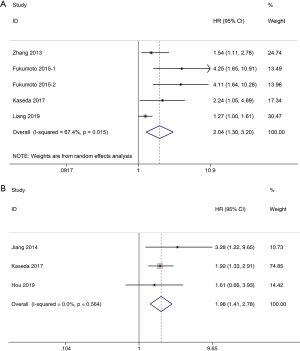
Four studies with a total of 1,338 patients compared OS between NSCLC patients with high preoperative D-dimer level and these with normal preoperative D-dimer level after surgery. Our meta-analysis found that NSCLC patients with high preoperative D-dimer level had a significantly worse OS than these with normal preoperative D-dimer level (random effects: HR =2.04; 95% CI: 1.30–3.20; P=0.002; I2=67.4%) (Figure 2A) after surgery. Three studies with a total of 816 patients compared DFS between patients with high preoperative D-dimer level and those with normal preoperative D-dimer level after surgical resection. And our meta-analysis found that NSCLC patients with high preoperative D-dimer level also had a significantly worse DFS than those with normal preoperative D-dimer level (fixed effects: HR =1.98; 95% CI: 1.41–2.78; P<0.001; I2=0.0%) (Figure 2B) after surgery. Significant heterogeneity was only observed during the analysis of OS.
Sensitivity analysis and publication bias
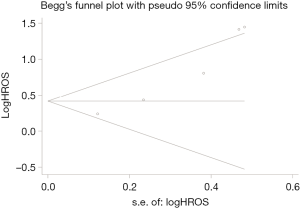
A sensitivity analysis was conducted by sequential removal of each study to evaluate the stability of our primary results based on OS. Sensitivity analysis found that sequential removal of each study did not change the result of above analysis (Figure 3). Publication bias was evaluated with a funnel plot for the analysis of OS. However, the funnel plot exhibited an asymmetrical appearance (Begg’s test: P=0.014; Egger’s test: P=0.007), indicating potential publication bias (Figure 4).
Discussion
D-dimer, which is the fibrinolytic degradation products of crosslinked fibrin, is generally utilized as a useful marker for the diagnosis of pulmonary embolism with high sensitivity but low specificity (9,10). Recent study found that D-dimer level in NSCLC patients was significantly higher than that of healthy controls (23) and the prevalence of high pretreatment D-dimer level in NSCLC patients was reported to be as high as about 67.9% (19). However, the impact of high preoperative D-dimer level on long-term prognosis of patients with surgically treated NSCLC remains to be determined. Hence, we conducted the first meta-analysis to figure out the prognostic value of high preoperative D-dimer level in patients with surgically treated NSCLC. In this meta-analysis, we finally included 6 cohort studies with a total of 1,817 patients with surgically treated NSCLC. Our meta-analysis found that high preoperative D-dimer level was significantly correlated with worse OS (HR =2.04; 95% CI: 1.30–3.20; P=0.002) and DFS (HR =1.98; 95% CI: 1.41–2.78; P<0.001) for NSCLC patients after surgical resection. Therefore, our meta-analysis adds to the evidence that high preoperative D-dimer level could serve as an independent unfavorable prognostic factor of patients with surgically treated NSCLC.
In malignancies, tumor cells could activate the coagulation system by producing procoagulant factors, such as proteins, lipids, and inflammatory cytokines, which could lead to a hypercoagulable state (24). Because of the enhanced procoagulant activity, the levels of fibrinogen and subsequent fibrin degradation products (such as D-dimer) were significantly increased in cancer patients (25), and therefore, high level of D-dimer could serve as an indicator of hypercoagulable status. Previous evidence showed that hypercoagulable status could greatly facilitate tumor growth, angiogenesis, tumor cell invasion, and metastasis (24,26). Therefore, it is reasonable that high level of D-dimer could significantly contribute to tumor aggressiveness and invasiveness. As a result, high level of D-dimer was found to be significantly correlated with advanced tumor stage, and more number of metastatic sites (18,27). Moreover, D-dimer level could also serve as a clinically important predictor for the positive lymph node involvement in operable NSCLC patients (28) and it was also found to gradually increase as terminal stage cancer patients approaching to death (29). In addition, the level of D-dimer was also correlated significantly with tumor biomarker level (such as carcinoembryonic antigen) (20) and performance status (30). It is reported that D-dimer level decreased after response to chemoradiotherapy but increased after disease progression, which suggested that D-dimer level change could also serve as a predictor for treatment efficacy and monitoring disease progression (31,32). Taken together, we believe that high preoperative level of D-dimer could serve as an independent unfavorable prognostic factor for NSCLC patients after surgical resection. However, more efforts should be made to elucidate the detailed interactive mechanisms between D-dimer level and lung cancer. As for clinical implications, we think that preoperative monitoring of D-dimer level for NSCLC patients intended for surgery is of great importance. And for NSCLC patients with high preoperative level of D-dimer intended for surgical resection, lowering D-dimer level with anticoagulant drugs such as low-molecular weight heparin may be considered, which may help not only prevent thromboembolism complications but also improve long-term survival (33). Moreover, postoperative monitoring of D-dimer level in NSCLC patients after surgery may be also incorporated into postoperative follow-up strategies, which may help with directing postoperative treatment strategies and predicting early recurrence. And for NSCLC patients with high postoperative D-dimer level, decreasing D-dimer level should also be recommended for both thromboembolism prevention and decreasing cancer recurrence.
There were several limitations in our meta-analysis. First, with only six retrospective cohort studies included in our analysis, the validity of our meta-analysis may be influenced due to small sample size and patient selection bias. Second, the cut-off value for defining high level of D-dimer varied among those studies, which may cause heterogeneities. Finally, potential heterogeneity and publication bias was observed during analysis, which could influence our results.
Conclusions
We conducted the first meta-analysis to investigate the prognostic value of high preoperative level of D-dimer in NSCLC patients after surgical resection. We found that high preoperative level of D-dimer was an independent predictor of poor OS and DFS in surgically treated NSCLC patients. Therefore, routinely monitoring D-dimer level in NSCLC patients intended for surgery may be recommended for daily practice. Further studies with appropriate adjustments, however, are needed to confirm and update our conclusions.
Supplementary
Acknowledgment
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gadgeel SM, Ramalingam SS, Kalemkerian GP. Treatment of lung cancer. Radiol Clin North Am 2012;50:961-74. [Crossref] [PubMed]
- Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727-40. [Crossref] [PubMed]
- Cruz C, Afonso M, Oliveiros B, et al. Recurrence and Risk Factors for Relapse in Patients with Non-Small Cell Lung Cancer Treated by Surgery with Curative Intent. Oncology 2017;92:347-52. [Crossref] [PubMed]
- Thakur MK, Gadgeel SM. Predictive and Prognostic Biomarkers in Non-Small Cell Lung Cancer. Semin Respir Crit Care Med 2016;37:760-70. [Crossref] [PubMed]
- Zhou YX, Yang ZM, Feng J, et al. High plasma D-dimer level is associated with decreased survival in patients with lung cancer: a meta-analysis. Tumour Biol 2013;34:3701-4. [Crossref] [PubMed]
- Ma X, Li Y, Zhang J, et al. Prognostic role of D-dimer in patients with lung cancer: a meta-analysis. Tumour Biol 2014;35:2103-9. [Crossref] [PubMed]
- Li W, Tang Y, Song Y, et al. Prognostic Role of Pretreatment Plasma D-Dimer in Patients with Solid Tumors: A Systematic Review and Meta-Analysis. Cell Physiol Biochem 2018;45:1663-76. [Crossref] [PubMed]
- Mavromatis BH, Kessler CM. D-dimer testing: the role of the clinical laboratory in the diagnosis of pulmonary embolism. J Clin Pathol 2001;54:664-8. [Crossref] [PubMed]
- Deng HY, Li G, Luo J, et al. MicroRNAs are novel non-invasive diagnostic biomarkers for pulmonary embolism: a meta-analysis. J Thorac Dis 2016;8:3580-7. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Zhang PP, Sun JW, Wang XY, et al. Preoperative plasma D-dimer levels predict survival in patients with operable non-small cell lung cancer independently of venous thromboembolism. Eur J Surg Oncol 2013;39:951-6. [Crossref] [PubMed]
- Jiang HG, Li J, Shi SB, et al. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol 2014;31:22. [Crossref] [PubMed]
- Fukumoto K, Taniguchi T, Usami N, et al. Preoperative plasma D-dimer level is an independent prognostic factor in patients with completely resected non-small cell lung cancer. Surg Today 2015;45:63-7. [Crossref] [PubMed]
- Kaseda K, Asakura K, Kazama A, et al. Prognostic significance of preoperative plasma D-dimer level in patients with surgically resected clinical stage I non-small cell lung cancer: a retrospective cohort study. J Cardiothorac Surg 2017;12:102. [Crossref] [PubMed]
- Hou C, Jiang F, Ma H, et al. Prognostic role of preoperative platelet, fibrinogen, and D-dimer levels in patients with non-small cell lung cancer: A multicenter prospective study. Thoracic Cancer 2019;10:304-11. [Crossref] [PubMed]
- Liang HG, Gao K, Jia R, et al. Prognostic significance of the combination of preoperative fibrinogen and the neutrophil-lymphocyte ratio in patients with non-small cell lung cancer following surgical resection. Oncol Lett 2019;17:1435-44. [PubMed]
- Qi Y, Fu J. Research on the coagulation function changes in non small cell lung cancer patients and analysis of their correlation with metastasis and survival. J buon 2017;22:462-7. [PubMed]
- Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol 2009;22:49-60. [Crossref] [PubMed]
- Kvolik S, Jukic M, Matijevic M, et al. An overview of coagulation disorders in cancer patients. Surg Oncol 2010;19:e33-46. [Crossref] [PubMed]
- Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 2013;11:223-33. [Crossref] [PubMed]
- Ge LP, Li J, Bao QL, et al. Prognostic and predictive value of plasma D-dimer in advanced non-small cell lung cancer patients undergoing first-line chemotherapy. Clin Transl Oncol 2015;17:57-64. [Crossref] [PubMed]
- Chen F, Wang MJ, Li J, et al. Plasma D-dimer value as a predictor of malignant lymph node involvement in operable non-small cell lung cancer. Tumour Biol 2015;36:9201-7. [Crossref] [PubMed]
- Zhang X, Liu ZQ, Zhang W, et al. A retrospective analysis of plasma D-dimer dynamic variation in terminal stage cancer patients: implications for disease progression. Int J Clin Exp Med 2014;7:2395-401. [PubMed]
- Wang Y, Wang Z. Predictive value of plasma D-dimer levels in patients with advanced non-small-cell lung cancer. Onco Targets Ther 2015;8:805-8. [Crossref] [PubMed]
- Antoniou D, Pavlakou G, Stathopoulos GP, et al. Predictive value of D-dimer plasma levels in response and progressive disease in patients with lung cancer. Lung Cancer 2006;53:205-10. [Crossref] [PubMed]
- Zhu LR, Li J, Chen P, et al. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol 2016;18:178-88. [Crossref] [PubMed]
- Falanga A, Vignoli A, Diani E, et al. Comparative assessment of low-molecular-weight heparins in cancer from the perspective of patient outcomes and survival. Patient Relat Outcome Meas 2011;2:175-88. [Crossref] [PubMed]