
Efficacy and safety of cinacalcet and active vitamin D in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease: a network meta-analysis
Introduction
Secondary hyperparathyroidism (SHPT) is a common complication in patients with chronic kidney disease (CKD). Generally, SHPT develops in stage 3 CKD with an estimated glomerular rate (eGFR) <60 mL/min/1.73 m2, and its prevalence increases as renal function deteriorates (1,2). SHPT represents the initially adaptive and finally maladaptive response of organisms to the disordered balance of calcium, phosphorus, and parathyroid hormone (PTH) levels and vitamin D metabolism in patients with CKD (3). Clinically, SHPT often leads to profound alterations in bone metabolism (4) and vascular (5,6) and valvular calcification (7), which are linked to increased risks of cardiovascular morbidity and mortality as well as all-cause mortality (8).
To improve PTH levels and restore disordered mineral metabolism in patients with CKD, the Kidney Disease Improving Global Outcomes 2017 clinical practice guideline update for CKD-mineral and bone disorder (MBD) recommends that patients with stage 5D CKD requiring PTH-lowering therapy should receive treatment with a calcimimetic agent, active vitamin D, or a combination of a calcimimetic agent and active vitamin D (9).
Cinacalcet, an orally administered calcimimetic agent, was approved by the US Food and Drug Administration in 2004 and by the European Committee for Medical Products for Human Use in 2005 to treat SHPT in patients on dialysis (10). Cinacalcet acts directly by activating calcium-sensing receptors (CaSRs) in the parathyroid gland (11). Upon binding CaSR, cinacalcet allosterically increases its sensitivity to extracellular calcium, thus suppressing PTH secretion without increasing serum calcium and phosphate levels (11,12).
Vitamin D can directly reduce PTH synthesis and secretion via its high affinity for vitamin D receptors (VDRs) in the parathyroid gland, further inhibiting parathyroid hyperplasia (13-15). However, the potent action of vitamin D that enhances intestinal calcium and phosphorus absorption often leads to hypercalcemia and hyperphosphatemia, adding to the already high risk of extraskeletal calcification. In addition, hypercalcemia can lead to oversuppression of PTH, resulting in low bone turnover or adynamic bone disease. Abnormally low bone formation results in defective bone mineralization, which limits the therapeutic dose of vitamin D (16,17).
Because cinacalcet and vitamin D act through distinct mechanisms, their combined application could lead to more effective control of PTH levels, and their offsetting effects on calcium and phosphate may reduce the risks of hypercalcemia and hyperphosphatemia that are increased by vitamin D monotherapy (13,18). A previous study revealed that cinacalcet combined with conventional therapy (phosphate binders and vitamin D) leads to significant reductions in the risk of fracture, cardiovascular hospitalization and mortality and thus has favorable effects on important clinical outcomes (19).
Previous systematic reviews or meta-analyses (20-25) have highlighted the efficacy and safety of cinacalcet and active vitamin D as monotherapies, while data on the combination of cinacalcet and active vitamin are lacking. In particular, there has been no network meta-analysis (NMA) comparing cinacalcet, active vitamin D and cinacalcet plus active vitamin D. Consequently, it remains unclear which treatment benefits patients with CKD-SHPT most. A pairwise comparison meta-analysis is inadequate to determine the superiority of a regimen. It is increasingly popular to use an NMA to assess medical interventions, especially because head-to-head comparisons are lacking. NMAs could provide an effective way to evaluate the relative effectiveness of all interventions and allow ranking of the interventions. Therefore, in the present study, we conducted an NMA to evaluate the efficacy and safety of cinacalcet, active vitamin D and cinacalcet plus active vitamin D.
Methods
Search strategy and selection criteria
A literature search was conducted in electronic databases by two independent reviewers (LH Ni and RN Tang). Multiple resources were searched to prevent selection bias, including the Cochrane Library, PubMed, EMBASE, Web of Science (WOS), Google Scholar, China National Knowledge Internet (CNKI) and Wanfang databases, covering all articles published up to November 2018. The following terms were used: “secondary hyperparathyroidism”, “SHPT”, “cinacalcet”, “vitamin D”, “randomized controlled trial” and “RCT”. We limited the studies included in this meta-analysis to randomized controlled trials (RCTs) evaluating the effectiveness and safety of cinacalcet and active vitamin D for the treatment of SHPT. The comparisons were cinacalcet and active vitamin D alone or cinacalcet plus vitamin D. We explored the effectiveness of treatment according to the following outcomes: all-cause mortality, hemodialysis (HD)-related patient mortality, 1-year mortality and the compliance rates of intact PTH (iPTH), blood calcium and blood phosphorus. We also collected adverse events, such as nausea, vomiting, hypocalcemia, hypercalcemia, muscle spasms and diarrhea.
Data extraction and quality assessment
Two investigators (Lihua Ni and Rining Tang) independently reviewed the articles, and disagreements were resolved by discussion and consensus. Using a standardized data collection form, we collected the following information from each study: the author, date of publication, eligibility criteria, summary of the baseline characteristics of the participants, number of participants in each arm at study onset and completion, duration of the trial, and therapeutic effects, including effectiveness and safety.
The quality of the included studies was evaluated using the Cochrane risk assessment tool (26,27). This scale included the method of randomization, double blinding, and a description of dropouts.
Statistical analysis
A conventional pairwise meta-analysis was performed with Review Manager (version 5.3, The Cochrane Collaboration), and an NMA was performed with STATA 13.1 (Stata Corporation, College Station, TX, USA). A value of P<0.05 was regarded as statistically significant. The pooled data were used to assess the efficacy and safety as indicated by the relative risk (RR) with a 95% confidence interval (CI), which was calculated based on the random effects model or fixed effects model for investigating treatment effects. A Z test was conducted to assess the significance of the overall effect size.
After constructing a heterogeneity matrix, the frequentist method was applied to the fitted meta-regression model. The model includes the basic parameters as covariates and assumes that heterogeneity is independent of the comparison between effect sizes in multiarm studies. Inconsistency refers to the differences between direct and various indirect effects estimated for the same comparison. For indirect comparisons, treatment effects of all treatment regimens were estimated by applying a two-stage NMA as follows: Firstly, the inconsistency test through node-splitting model and the fitting consistency model or inconsistency model were performed and presented through the network command. However, due to the inability of the NMA to perform loop comparison, inconsistency test would not be performed. So, fitting consistency model was performed and presented through the network command. We estimated the probability of a treatment being ranked at a specific position according to the outcome using a “network rank”. The results of the NMA with regard to therapeutic effect are shown in a forest plot for pairwise comparisons in the network.
Results
Screening and inclusion of studies
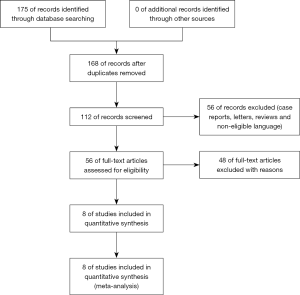
In the present meta-analysis, 168 relevant studies were identified, and their titles and abstracts were reviewed. Subsequently, 112 studies were excluded, as they were case reports, letters, reviews or articles written in a language other than English or Chinese. After full-text review of the remaining studies, 48 studies were excluded due to their study design. Specifically, 12 studies were retrospective studies, 17 studies were cell or animal studies, 13 studies were irrelevant interventions, and 6 studies were excluded for other reasons. Finally, 8 RCTs (28-35) with 1,443 patients were eligible for this meta-analysis. The screening and inclusion process are presented in Figure 1, and the baseline characteristics of the included studies are summarized in Table 1. The quality of the studies is shown in Figure 2.
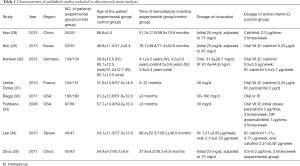
Full table

Pairwise meta-analysis
Long-term mortality
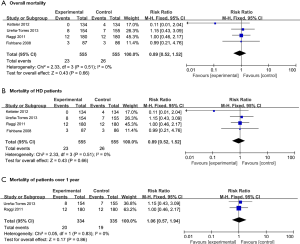
Data available regarding the survival outcomes were limited, although some studies reported 5-year mortality rates. Therefore, we could only analyze all-cause mortality, mortality of HD patients and 1-year mortality as survival outcomes. The all-cause mortality rate means that the mortality rate of all causes of death and HD-related mortality are related to the mortality rate of HD patients.
In total, 1,110 patients in four RCTs were included in the analysis of survival outcomes. The results revealed that cinacalcet plus active vitamin D did not significantly improve survival compared with active vitamin D monotherapy (all-cause mortality: RR =0.89, 95% CI: 0.52–1.52, P=0.66; mortality of HD patients: RR =0.89, 95% CI: 0.52–1.52, P=0.66; 1-year mortality: RR =1.06, 95% CI: 0.57–1.94, P=0.86, Figure 3).
The efficacy of cinacalcet plus active vitamin D
Figure 4 presents the forest plots for the meta-analysis of the efficacy of cinacalcet plus active vitamin D compared with active vitamin D alone in patients with SHPT. The compliance rates of serum indicators were selected to evaluate the efficacy. The standard range is based on the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (36).
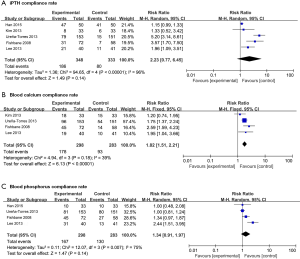
Our research revealed that compared with active vitamin D alone, cinacalcet plus active vitamin D significantly improved the blood calcium compliance rate (RR =1.82, 95% CI: 1.51–2.21, P<0.00001). However, no significant differences were found in the other indicators (iPTH: RR =2.23, 95% CI: 0.77–6.45, P=0.14 and blood phosphorus compliance rate: RR =1.34, 95% CI: 0.91–1.97, P=0.14).
Safety
Safety is an important aspect of drug evaluations. To address safety, we compared the toxicities of cinacalcet plus active vitamin D with those of active vitamin D alone. This pairwise meta-analysis evaluated the following most commonly reported toxicities: nausea, vomiting, hypercalcemia, hypocalcemia, muscle spasms and diarrhea. Cinacalcet plus active vitamin D and active vitamin D alone had no significant differences in the rates of toxicities (nausea: RR =4.15, 95% CI: 0.47–36.54, P=0.20; hypercalcemia: RR =0.51, 95%: 0.01–37.66, P=0.76; hypocalcemia: RR =5.77, 95% CI: 0.17–194.05, P=0.33; muscle spasms: RR =1.13, 95% CI: 0.66–1.95, P=0.65; diarrhea: 1.13, 95% CI: 0.72–1.80, P=0.59), except vomiting, which was significantly more common in patients receiving cinacalcet plus active vitamin D than in those receiving active vitamin D alone (RR =2.07, 95% CI: 1.18–3.65, P=0.01; Figure 5).
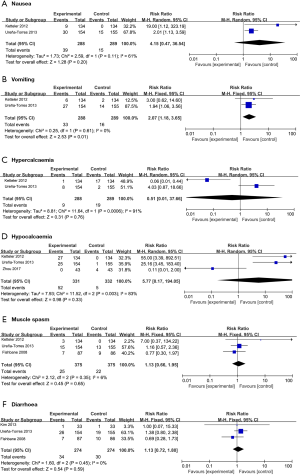
NMA (Figures 6-11)
Evidence network
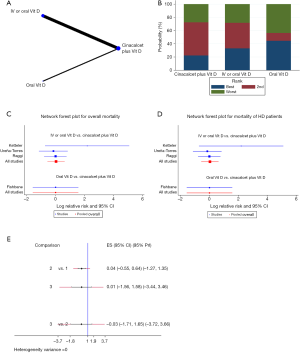
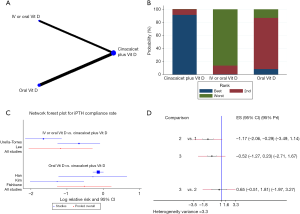
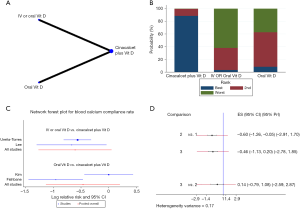
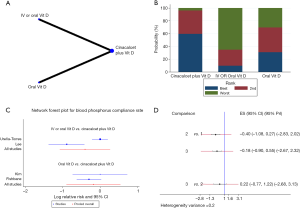
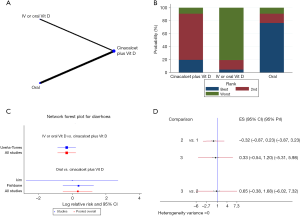
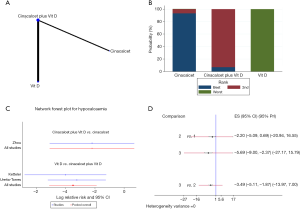
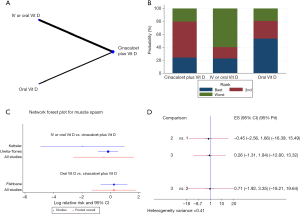
The evidence network is displayed in Figures 6A,7A,8A,9A,10A,11A. Connecting lines indicate direct comparisons between the two interventions, and pairs of interventions without connections were compared indirectly through an NMA. The width of each line represents the number of trials. The size of each node indicates the overall sample size of the intervention.
Evaluating and presenting assumptions of the NMA
The present NMA had no triangular loop; therefore, there was no source of inconsistency. The NMA was based on the specific treatment of the control group, including the routes of administration of active vitamin D and different drugs. The routes of administration of active vitamin D included intravenous (IV) administration and oral administration. The different drugs in the control group were mainly active vitamin D alone or cinacalcet alone.
Administration of active vitamin D
Only one original study (35) included oral administration of vitamin D, and the rest were oral or IV administration. The four RCTs that reported the mortality of patients were included in this NMA. Compared with oral or IV administration of vitamin D, the solely oral administration of active vitamin D may increase mortality (Figure 6B). Further statistical tests were conducted on this possibility. We found that the difference in mortality was not statistically significant [RR =−0.03, 95% CI: −1.71 to 1.65; 95% prediction interval (PrI): −3.72 to 3.66]. It is worth noting that compared with active vitamin D alone, cinacalcet plus active vitamin D reduced mortality (RR =0.04, 95% CI: −0.55 to 0.64; Figure 6C,D,E), but the reliability of this promising result is worthy of further scrutiny, considering that the PrI crossed the invalid line (95% PrI: −1.27 to 1.35; Figure 6E). The results were consistent with those of the pairwise meta-analysis (Figure 3A,B).
Furthermore, an NMA was performed to explore the compliance rates of iPTH, blood calcium, blood phosphorus and Ca × P. Cinacalcet plus active vitamin D appeared to increase the efficacy of the treatment compared with that of either agent alone, and the oral administration of active vitamin D increased compliance rates compared with those of oral or IV vitamin D administration (Figures 7B,8B,9B,10B). However, the results of the consistency model indicated that there were no differences in compliance between the two modes of administration (compliance rate of iPTH: RR =0.65, 95% CI: −0.51 to 1.81, 95% PrI: −1.97 to 3.27, Figure 7C,D; compliance rate of blood calcium: RR =0.14, 95% CI: −0.79 to 1.08, 95% PrI: −2.59 to 2.87, Figure 8C,D; compliance rate of blood phosphorus: RR =0.22, 95% CI: −0.77 to 1.22, 95% PrI: −2.68 to 3.13, Figure 9C,D). In addition, there were no differences in toxicities between the administration methods (diarrhea: RR =0.65, 95% CI: −0.38 to 1.68, 95% PrI: −6.02 to 7.32, Figure 10B,D; muscle spasms: RR =0.71, 95% CI: −1.92 to 3.35, 95% PrI: −18.21 to 19.64, Figure S1).
Different drugs in the control group
In the pairwise meta-analysis, all comparisons were based on cinacalcet plus active vitamin D compared with active vitamin D alone in patients with SHPT; control groups receiving cinacalcet alone were very rare. An NMA was performed to explore the incidence of hypocalcemia when the control group received cinacalcet alone. Our NMA indicated that compared with the other two treatments, cinacalcet monotherapy increased the risk of hypocalcemia (cinacalcet plus active vitamin D vs. cinacalcet: RR =−2.20, 95% CI: −5.09 to 0.69; active vitamin D vs. cinacalcet: RR =−5.69, 95% CI: −9.00 to −2.37, Figure 11B,D), even though the risk of hypocalcemia due to the administration of cinacalcet plus active vitamin D was higher than that of active vitamin D alone (RR =−3.49, 95% CI: −5.11 to −1.87, Figure 11D). However, the results should be treated with caution because the PrI crossed the invalid line (cinacalcet plus active vitamin D vs. cinacalcet: 95% PrI: −20.94 to 16.55; active vitamin D vs. cinacalcet: 95% PrI: −27.17 to 15.79; active vitamin D vs. cinacalcet plus active vitamin D: 95% PrI: −13.97 to 7.00, Figure 11D). Given these contradictory results, we speculate that if more high-quality RCTs emerge in the future, the existing results may be overturned.
Publication bias
Only eight original studies were included in this meta-analysis, and the number of RCTs was lower for some of the specific analyses; therefore, we did not test for publication bias in this study.
Discussion
This meta-analysis aimed to evaluate the compliance of iTPH, Ca, P, etc., and the mortality and safety of cinacalcet plus active vitamin D and active vitamin D alone. In addition, we used an NMA to estimate the safety and efficacy of three treatment regimens (cinacalcet alone, active vitamin D alone and cinacalcet plus activated vitamin D) though direct and indirect statistical comparisons based on all available information from the included RCTs.
Cinacalcet and vitamin D as monotherapies or in combination are common treatments for SHPT in patients with CKD, aimed at achieving clinically acceptable levels of PTH and maintaining control of calcium and phosphorus levels. Numerous trials have documented the efficacy of these three regimens for the treatment of SHPT (20,30,37-40). However, there are currently two challenging questions facing the medical community with regard to these treatments. Is there enough evidence to support the claim that cinacalcet is more effective than vitamin D and its derivatives? Is the combination of the two drugs more effective than each of the two drugs alone? A consensus regarding the former question has begun to form in clinical research and meta-analyses (24), but the latter issue has not been resolved. Therefore, the first step in our research was to compare the efficacy and safety of the combination therapy with those of active vitamin D alone. Our research revealed that compared with active vitamin D alone, cinacalcet plus active vitamin D significantly improved the blood calcium compliance rate, but there was no significant improvement in long-term survival. Next, our study investigated which treatment benefits patients the most and the advantages or disadvantages of the three treatment regimens (cinacalcet alone, active vitamin D alone and cinacalcet plus active vitamin D). Through direct and indirect comparisons, the results of our NMA revealed the following two positive results: (I) compared with oral or IV administration of vitamin D, the solely oral administration of active vitamin D increased mortality; (II) cinacalcet alone increased the risk of hypocalcemia, cinacalcet plus active vitamin D conferred a higher risk of hypocalcemia than did active vitamin D monotherapy; in addition, cinacalcet monotherapy conferred a higher risk of hypocalcemia than did cinacalcet plus active vitamin D. However, the two positive results should be treated with caution because the PrI crossed the invalid line.
The following study limitations should also be acknowledged: only English and Chinese language studies were included, which might have led to potential publication bias, and the exclusion of unpublished data is generally associated with an overestimation of the true effect. Regardless of the route of administration, the total dose of vitamin D was not the same in the control group. This phenomenon may be caused by many factors. We believe that different centers refer to different treatment guidelines or experience in treatment. In addition, when both groups had low mortality rates, the results of the differences needed to be treated with caution. However, the following limitation was the most noteworthy. The NMA was based on the specific treatment of the control group, such as the administration of active vitamin D alone. Therefore, the experimental groups also needed to be divided into different intervention groups. It is controversial to have regarded combination therapy as an intervention. To achieve an indirect comparison of single drugs, our approach may be effective.
Our research suggests that compared with active vitamin D alone, cinacalcet plus active vitamin D may significantly improve the blood calcium compliance rate but cannot prolong survival. In addition, compared with monotherapy, combination therapy increases the risk of vomiting. This pairwise meta-analysis and NMA provided a comprehensive evaluation of the currently utilized CKD-SHPT treatments. This NMA identified some highly ranked interventions through analyses that were included in a small number of trials and that merit further examination on a larger scale in the context of well-designed RCTs.
Acknowledgments
Funding: This study was funded by grants from the National Natural Science Foundation of China (31571186, 81770735, 81370919, 81470997), The Medical Talents of Jiangsu Province (ZDRCA2016079), the Natural Science Foundation of Jiangsu Province (BK20161437), Clinical Medicine Science and Technology Project-Clinic Research Center of Jiangsu Province (BL2014080), and Jiangsu Commission of Health “Six One Project” (LGY2018097).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- De Boer IH, Gorodetskaya I, Young B, et al. The severity of secondary hyperparathyroidism in chronic renal insufficiency is GFR-dependent, race-dependent, and associated with cardiovascular disease. J Am Soc Nephrol 2002;13:2762-9. [Crossref] [PubMed]
- Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int 2007;71:31-8. [Crossref] [PubMed]
- Friedl C, Zitt E. Vitamin D prohormone in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Int J Nephrol Renovasc Dis 2017;10:109-22. [Crossref] [PubMed]
- Malluche H, Faugere MC. Renal bone disease 1990: an unmet challenge for the nephrologist. Kidney Int 1990;38:193-211. [Crossref] [PubMed]
- Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002;39:695-701. [Crossref] [PubMed]
- Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 2000;342:1478-83. [Crossref] [PubMed]
- Ribeiro S, Ramos A, Brandao A, et al. Cardiac valve calcification in haemodialysis patients: role of calcium-phosphate metabolism. Nephrol Dial Transplant 1998;13:2037-40. [Crossref] [PubMed]
- Covic A, Kothawala P, Bernal M, et al. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009;24:1506-23. [Crossref] [PubMed]
- Ketteler M, Block GA, Evenepoel P, et al. Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: Synopsis of the Kidney Disease: Improving Global Outcomes 2017 Clinical Practice Guideline Update. Ann Intern Med 2018;168:422-30. [Crossref] [PubMed]
- Nagano N. Pharmacological and clinical properties of calcimimetics: calcium receptor activators that afford an innovative approach to controlling hyperparathyroidism. Pharmacol Ther 2006;109:339-65. [Crossref] [PubMed]
- Nemeth EF, Steffey ME, Hammerland LG, et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci U S A 1998;95:4040-5. [Crossref] [PubMed]
- Zitt E, Jager C, Rosenkranz AR, et al. Effective use of cinacalcet for the treatment of secondary hyperparathyroidism in Austrian dialysis patients--results of the Austrian cohort of the ECHO study. Wiene klin Wochenschr 2011;123:45-52.
- Brown AJ. Vitamin D analogs for secondary hyperparathyroidism: what does the future hold? J Steroid Biochem Mol Biol 2007;103:578-83. [Crossref] [PubMed]
- Seeherunvong W, Nwobi O, Abitbol CL, et al. Paricalcitol versus calcitriol treatment for hyperparathyroidism in pediatric hemodialysis patients. Pediatr Nephrol 2006;21:1434-9. [Crossref] [PubMed]
- Chanchlani R, Ackerman S, Piva E, et al. Intraperitoneal Calcitriol for Treatment of Severe Hyperparathyroidism in Children with Chronic Kidney Disease: A Therapy Forgotten. Perit Dial Int 2016;36:688-90. [Crossref] [PubMed]
- Marcinowska-Suchowierska E, Kupisz-Urbańska M, Łukaszkiewicz J, et al. Vitamin D Toxicity-A Clinical Perspective. Front Endocrinol (Lausanne) 2018;9:550. [Crossref] [PubMed]
- Kilpatrick RD, Danese MD, Belozeroff V, et al. The association of vitamin D use with hypercalcemia and hyperphosphatemia in hemodialysis patients: a case-crossover study. Pharmacoepidemiol Drug Saf 2011;20:914-21. [PubMed]
- Ritter C, Miller B, Coyne DW, et al. Paricalcitol and cinacalcet have disparate actions on parathyroid oxyphil cell content in patients with chronic kidney disease. Kidney Int 2017;92:1217-22. [Crossref] [PubMed]
- Cunningham J, Danese M, Olson K, et al. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int 2005;68:1793-800. [Crossref] [PubMed]
- Greeviroj P, Kitrungphaiboon T, Katavetin P, et al. Cinacalcet for Treatment of Chronic Kidney Disease-Mineral and Bone Disorder: A Meta-Analysis of Randomized Controlled Trials. Nephron 2018;139:197-210. [Crossref] [PubMed]
- Wang G, Liu H, Wang C, et al. Cinacalcet versus Placebo for secondary hyperparathyroidism in chronic kidney disease patients: a meta-analysis of randomized controlled trials and trial sequential analysis. Sci Rep 2018;8:3111. [Crossref] [PubMed]
- van der Plas WY, Dulfer RR, Engelsman AF, et al. Effect of parathyroidectomy and cinacalcet on quality of life in patients with end-stage renal disease-related hyperparathyroidism: a systematic review. Nephrol Dial Transplant 2017;32:1902-8. [Crossref] [PubMed]
- Sekercioglu N, Busse JW, Sekercioglu MF, et al. Cinacalcet versus standard treatment for chronic kidney disease: a systematic review and meta-analysis. Ren Fail 2016;38:857-74. [Crossref] [PubMed]
- Sekercioglu N, Busse JW, Mustafa RA, et al. Cinacalcet versus standard treatment for chronic kidney disease: a protocol for a systematic review and meta-analysis. Syst Rev 2016;5:2. [Crossref] [PubMed]
- Palmer SC, Nistor I, Craig JC, et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med 2013;10:e1001436. [Crossref] [PubMed]
- Schünemann HJ, Oxman AD, Brozek J, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evid Based Med 2008;13:162-3. [Crossref] [PubMed]
- Deeks JJ, Altman DG. Chapter 9. Analysing Data and Undertaking Meta-Analyses. Cochrane Handbook for Systematic Reviews of Interventions Cochrane Book, 2011:342-50.
- Han YY, Wang T, Zhang WY, et al. Clinical observation of calcitriol combined with cinacalcet in the treatment of hemodialysis patients with secondary hyperparathyroidism. Drugs & Clinic 2015;30:1451-4.
- Kim HJ, Kim H, Shin N, et al. Cinacalcet lowering of serum fibroblast growth factor-23 concentration may be independent from serum Ca, P, PTH and dose of active vitamin D in peritoneal dialysis patients: a randomized controlled study. BMC nephrol 2013;14:112. [Crossref] [PubMed]
- Ketteler M, Martin KJ, Cozzolino M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: study design and baseline characteristics of the IMPACT SHPT study. Nephrol Dial Transplant 2012;27:1942-9. [Crossref] [PubMed]
- Ureña-Torres P, Bridges I, Christiano C, et al. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol Dial Transplant 2013;28:1241-54. [Crossref] [PubMed]
- Raggi P, Chertow GM, Torres PU, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant 2011;26:1327-39. [Crossref] [PubMed]
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718-25. [Crossref] [PubMed]
- Lee YT, Ng HY, Kuo CC, et al. Comparison between calcitriol and calcitriol plus low-dose cinacalcet for the treatment of moderate to severe secondary hyperparathyroidism in chronic dialysis patients. Nutrients 2013;5:1336-48. [Crossref] [PubMed]
- Zhou L, Zhao WT, Ye T, et al. Curative effect of cinacalcet combined with active vitamin D in the treatment of hyperparathyroidism secondary to maintenance hemodialysis. Chinese Journal of Integrated Traditional Chinese and Western Medicine 2017;24:650-3.
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1-201. [Crossref] [PubMed]
- Sharma A, Marshall TS, Khan SS, et al. Cost effectiveness of paricalcitol versus cinacalcet with low-dose vitamin D for management of secondary hyperparathyroidism in haemodialysis patients in the USA. Clin Drug Investig 2014;34:107-15. [Crossref] [PubMed]
- Plosker GL. Cinacalcet: a pharmacoeconomic review of its use in secondary hyperparathyroidism in end-stage renal disease. PharmacoEconomics 2011;29:807-21. [Crossref] [PubMed]
- Cheng J, Zhang W, Zhang X, et al. Efficacy and safety of paricalcitol therapy for chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol 2012;7:391-400. [Crossref] [PubMed]
- Han T, Rong G, Quan D, et al. Meta-analysis: the efficacy and safety of paricalcitol for the treatment of secondary hyperparathyroidism and proteinuria in chronic kidney disease. Biomed Res Int 2013;2013:320560. [Crossref] [PubMed]


