
High methylation of ZNF582 in cervical adenocarcinoma affects radiosensitivity and prognosis
Introduction
Cervical cancer ranks fourth in terms of both incidence and mortality in women globally (1). Over 85% of new cases and 90% of cervical cancer deaths occur in developing countries (1,2). In China, cervical cancer is still a huge clinical burden with high incidence (7.5/100,000) and mortality (3.4/100,000) rates (3). In terms of the tissue type affected, cervical adenocarcinoma (CAC) is the second most common to squamous cell carcinoma (SCC). The histology of adenocarcinoma is an independent significant risk factor for recurrence and survival (4,5). Both CAC and SCC are HPV-related cancers (6). Although early screening and the use of HPV vaccine have greatly decreased the incidence of cervical cancer in some countries and regions, the frequency of CAC has increased in many countries, particularly in younger women, and it now accounts for 20–25% of cervical cancer in developed countries (7). About 2/3 (60.3%) of CAC patients are younger than 50 years old, of which more than half (57.38%) are younger than 40 (8). Existing screening methods such as cytology-based tests are not ideal for CAC early detection because of low sensitivity and low reproducibility (9-11). The increased frequency and poor prognosis of CAC call for new biomarkers to help early diagnosis, treatment, and prognosis monitoring, and to enable individualized treatment and services.
Our understanding of DNA methylation patterns as potential biomarkers for diagnosis, prognosis, personalized therapy, and disease management is just starting to emerge. As a biomarker to detect cervical SCC, DNA methylation has several advantages over conventional cytology-based tests. Our previous studies showed that the ZNF582 methylation test improves on current cervical cancer screening efficacy and reduces unnecessary referral for colposcopy and biopsy by up to 60% (12-14), but how it performs in CAC is unclear. Whether DNA methylation can predict clinical outcome of CAC is not known. To investigate this problem, we conducted a series of follow-up investigations based on our previous studies, to investigate whether methylation of ZNF582 might contribute to the early diagnosis, radiochemotherapy sensitivity and prognosis prediction in CAC.
Methods
Study design and specimen collection
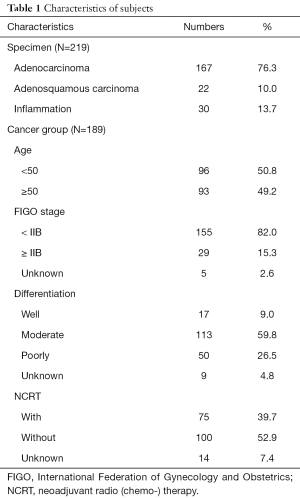
This was a retrospective clinical investigation studying methylation of ZNF582 in CAC. We enrolled 232 patients from Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, between 2013 and 2015. Among these patients, 202 were diagnosed with adenocarcinoma, 22 were diagnosed with adenosquamous carcinoma, and 30 were non-cancer specimens (inflammation cases). After evaluation, 13 subjects were excluded (six specimens lacked sufficient clinical information and seven had very low DNA concentration). This left 219 to be subjected to gene methylation studies (Table 1). We tracked the prognoses of these patients.
Full table
DNA preparation, bisulfite conversion, and quantitative methylation-specific polymerase chain reaction (qMSP)
All the methylation tests were performed in an ISO17025-certified laboratory (iStat Biomedical Co., Ltd., New Taipei City, Taiwan). Briefly, paraffin-embedded cervical tissues were deparaffinized and genomic DNA (gDNA) samples were then extracted and bisulfite-converted with the use of an EpiGene™ nucleic acid extraction kit and an EpiGene™ bisulfite conversion kit (iStat Biomedical Co., Ltd., New Taipei City, Taiwan). Quantitative methylation-specific PCR (qMSP) was later performed to determine the methylation level of ZNF582 (ZNF582m) using the TaqMan Probe system in a Light Cycler LC480 system (Roche Applied Science, Penzberg, Germany). Specific primers and probes for qMSP were published in our previous study (13). Hypermethylation (positive) was inferred where the delta Cp value was smaller than 11 (the cut-off value; Figure 1). The registered CaSki and A375 cancer cell lines were used as methylated and unmethylated controls to ensure the quality of the bisulfite conversion and qPCR processes.
Transfection of ZNF582
Hela cells were transfected with 20 µg of ZNF582 expression vectors (Vigene biosciences, NM_144690) using ViaFect Transfection Reagent (Promega Corporation).
Western blotting, immunohistochemistry, and immunofluorescence assays
Crude cellular protein was loaded onto an SDS-PAGE gel for protein separation and subsequently transferred to nitrocellulose membranes. After incubation in blocking solution (5% nonfat milk), the membranes were probed using primary antibodies against ZNF582 (Biorbyt, 1:1,000 dilution) overnight at 4 °C. They were detected using a Bio-Rad imaging system after incubation with horseradish peroxidase-conjugated secondary antibodies for 1.5 h. For immunohistochemistry and immunofluorescence, the slides were treated with the primary antibody against ZNF582 (Biorbyt, 1:200 dilution) at room temperature for 1 h after hydration and blocking, and then incubated with the secondary antibody for 10 min and examined using a light microscope or fluorescence microscope.
Statistical analysis
The cutoff values for defining the methylation status of the ZNF582 gene were generated from data gathered from 204 subjects, of which 30 were non-cancer controls and 174 were cancer patients. In this study, ROC curve analysis was used to determine which M-index of the target gene ZNF582 would have the highest accuracy for discerning worse cases of CAC from milder cases. An optimal cut-off value for the biomarkers was determined by the Youden index (15) from the correlations between M-index and case severity. The relationship between ZNF582 methylation and clinical pathological parameters of patients was analyzed using the Mann-Whitney and Dunnett tests. The data were analyzed using Graphpad Prism 5. The Kaplan-Meier method was used to estimate disease-free survival and overall survival (DFS and OS). We calculated DFS from treatment to the date of the first relapse at any site or death from any cause, whichever occurred first, and OS to death from any cause, and calculated hazard ratios (HRs) with multivariate Cox regression analysis.
Results
Performance of ZNF582 methylation as a biomarker of CAC
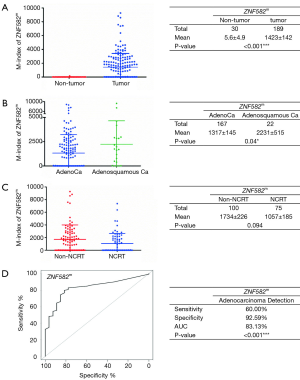
Among our 219 cervical specimens, 189 were cancer tissues (167 adenocarcinoma and 22 adenosquamous carcinoma), and 30 were non-cancer tissues (inflammations) (Table 1). The ZNF582 methylation index was significantly higher in the cancer group than in the non-cancer groups (Figure 2A). The methylation index was also higher in the adenosine squamous carcinoma group than in the adenocarcinoma group (Figure 2B). Among the cancer patients, 75 were subjected to neoadjuvant chemoradiotherapy (NCRT) while 100 were not treated (Table 1). The results showed that ZNF582 methylation level was reduced in the NCRT group compared with that in the non-NCRT patients (Figure 2C).
To determine the clinical application of a ZNF582 methylation assay, and to obtain a suitable cutoff value for this assay, we calculated ROC curves and area under the curve (AUC) values. The sensitivity and specificity of ZNF582 were 60.00% and 92.59% respectively. The cutoff value of delta Cp was 11, and the AUC of ZNF582 was 83.13% (Figure 2D). Methylation level of ZNF582 was independent of differentiation, depth of invasion, tumor size, and FIGO stage (Figure S1).
ZNF582m-positive CAC patients have better prognosis
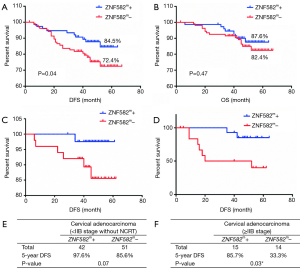
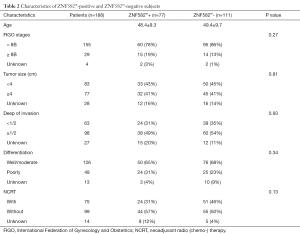
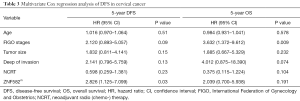
We further investigated whether the methylation level of ZNF582 influenced prognosis in CAC patients. After 5 years’ follow-up, we collated 188 patients’ prognostic information. Characteristics of ZNF582m-positive and ZNF582m-negative patients are shown in Table 2. Negative status for ZNF582 methylation was found to be an independent predictor of recurrence and survival [P=0.03, HR =2.826, 95% confidence interval (CI): 1.125–7.099; Table 3].
Full table
Full table
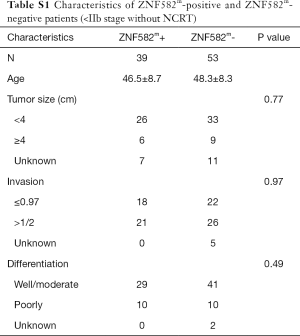
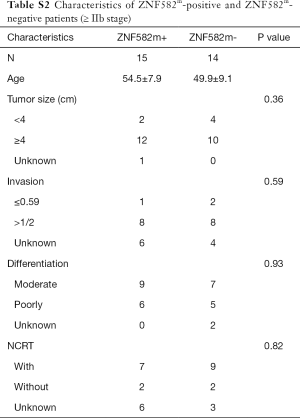
Five-year DFS rates were significantly higher in ZNF582m-positive patients than in ZNF582m-negative patients (84.5% vs. 72.4%; P=0.04) (Figure 2A), but there was no obvious difference in OS between these two groups (Figure 2B). For early stage (< IIB stage) patients without NCRT, ZNF582m-positive status was also associated with better prognosis, with an impressive 5-year DFS rate of 97.6% (Figure 2C). ZNF582m-negative patients with late-stage cancers (stage ≥ IIB) showed a very low 5-year DFS rate of 33.3% (Figure 2D). Characteristics of ZNF582m-positive and ZNF582m-negative patients are shown in Tables S1 and S2, respectively. The results indicated that ZNF582m-positive patients have better prognosis.
Full table
Full table
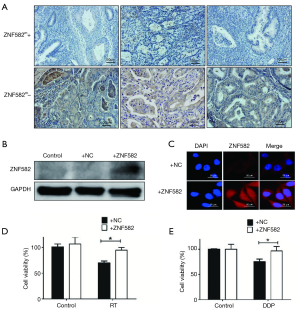
ZNF582m-negative status was correlated with high ZNF582 protein expression, and ZNF582 overexpression could increase radiation and chemotherapy resistance in Hela cells
To investigate the mechanism by which ZNF582 methylation affects the prognosis of CAC, we tested the association between ZNF582 protein expression and gene methylation in CAC tissues. We found that negative ZNF582 gene methylation status was correlated with high ZNF582 protein expression, and positive ZNF582 gene methylation status was correlated with low ZNF582 protein expression (Figure 3A). Further in vitro studies found that overexpression of ZNF582 protein in HeLa cells (Figure 3B,C) could increase their resistance to radiation (Figure 3D) and cisplatin treatment (Figure 3E).
Discussion
Several recent studies have found an increase in the incidence of CAC, particularly among younger women; for example in the United States (16,17), Sweden (18), and Italy (19). In the current study, we demonstrated a higher ZNF582 methylation level in cervical CAC compared with that in non-cancerous inflamed tissue (Figure 1A). Our data showed that ZNF582 methylation level reached 60.00% sensitivity and 92.59% specificity for the detection of CAC (Figure 1D), suggesting that the ZNF582 gene is appropriate for CAC detection. We also showed that ZNF582 methylation could contribute to differential diagnosis between adenocarcinoma and adenosine carcinoma (Figure 1B). Our third major finding was that ZNF582 methylation level was significantly reduced in patients who received NCRT compared with those who did not (Figure 1C). A good monitoring tool has always been crucial for understanding cancer treatment efficacy. Further study of ZNF582 methylation before and after NCRT, and investigation of other possible applications of ZNF582 methylation for monitoring cancer treatment efficacy, are warranted.
It is well known that CAC is radio-resistant and chemo-resistant, and its prognosis is worse than cervical SCC. In China, the prognosis of CAC is poor even for patients with early-stage disease (stage IB–IIB) (20), highlighting the need for closer attention to CAC. Our results showed the potential of ZNF582 methylation level as a biomarker for monitoring CAC treatment and prognosis, and this has not been considered elsewhere to our knowledge. ZNF582 methylation-positive patients have better prognosis in CAC (Figure 2A), suggesting that ZNF582 gene methylation has potential for 5-year DFS outcome prediction, in addition to being a prospective screening biomarker for early stage (< IIB) disease. Overall, our results suggest that ZNF582 methylation may play an important role in the molecular pathogenesis, clinical cancer progression, and prognosis of CAC.
Gene methylation is a regulatory epigenetic modification, regulated by DNA methyltransferase (DNMT) (21,22). Methylation can contribute to cancer development by modifying DNA base pairing patterns, resulting in stable mismatches that lead to carcinogenesis (23). Aberrant DNA methylation is associated with different types of cancer, such as cervical cancer (14,24,25), colorectal cancer (26), and lung cancer (27). Gene methylation can function as a negative regulator of gene expression, and have tumor-suppressive or oncogenic effects, potentially playing important roles in cancer progression. Prior to this study, the relationship between ZNF582 gene methylation and protein expression was unclear. We showed, for the first time, that ZNF582 gene hypermethylation correlates with low protein expression, and that negative ZNF582 gene methylation correlates with high protein expression (Figure 3A). Furthermore, we found that overexpression of ZNF582 protein increases resistance of Hela cells to radiation and chemotherapy (Figure 3D,E). Resistance to radiotherapy and chemotherapy is the main cause of poor prognosis in patients with CAC. These data may explain why ZNF582m-negative patients have a poor prognosis.
In our previous study, we reported 100% detection of ZNF582 methylation in SCC tissue (13), but in this study we found lower efficiency in CAC tissues. One possible reason for this is that HPV 16, which causes SCC, is much more prevalent than HPV 18, which causes CAC (28,29), and 20–40% of CAC is not associated with HPV infection (30). The second reason is that the sensitivity and accuracy of gene methylation detection in paraffin samples are not as good as in fresh-frozen specimens (31). For clinical applications, cervical scrapings rather than cervical tissues are more logical and feasible for methylation analysis and cancer detection. Whether the degree of methylation in cervical scrapings is consistent with cervical tissues requires further study.
In conclusion, this study suggests that ZNF582 methylation level may be useful as a biomarker for CAC detection. In addition, ZNF582 methylation may prove to be an important prognostic marker for CAC and may be a potential tool for monitoring the sensitivity of radiation and chemotherapy treatment.
Acknowledgments
The authors would like to thank all the members of Jing Wang laboratory, iStat Biomedical Co., Ltd and Hunan Hongya Gene Technology Co., Ltd.
Funding: This work was supported by the clinical research center in gynecologic cancer, Hunan Cancer Hospital, and the National Natural Science Foundation of China (2016YFC1303703), and Foundation from Social development science and technology division (2018SK2121, kq1801104). The kits were supported by iStat Biomedical Co. Ltd and Hunan Hongya Gene Technology Co., Ltd.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Hunan Cancer Hospital ethics committee {project number: 2015[01]} and the Chinese Clinical Trial Registry (registration number: ChiCTR1800018931).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Bermudez A, Bhatla N, Leung E. Cancer of the cervix uteri. Int J Gynaecol Obstet 2015;131 Suppl 2:S88-95. [Crossref] [PubMed]
- Wang SM, Qiao YL. Implementation of cervical cancer screening and prevention in China--challenges and reality. Jpn J Clin Oncol 2015;45:7-11. [Crossref] [PubMed]
- Nakanishi T, Ishikawa H, Suzuki Y, et al. A comparison of prognoses of pathologic stage Ib adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol 2000;79:289-93. [Crossref] [PubMed]
- Galic V, Herzog TJ, Lewin SN, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol 2012;125:287-91. [Crossref] [PubMed]
- An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol 2005;18:528-34. [Crossref] [PubMed]
- Fujiwara H, Yokota H, Monk B, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for cervical adenocarcinoma. Int J Gynecol Cancer 2014;24:S96-101. [Crossref] [PubMed]
- Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer 2009;125:525-9. [Crossref] [PubMed]
- Mbiguino A, Menezes J. Purification of human respiratory syncytial virus: superiority of sucrose gradient over percoll, renografin, and metrizamide gradients. J Virol Methods 1991;31:161-70. [Crossref] [PubMed]
- Powell A, Cohen PA, Spilsbury K, et al. RANZCOG Fellows' adherence to guidelines following cytological prediction of cervical adenocarcinoma-in-situ: Cause for concern? Aust N Z J Obstet Gynaecol 2019;59:294-300. [Crossref] [PubMed]
- Austin RM, Onisko A, Zhao C. Enhanced Detection of Cervical Cancer and Precancer Through Use of Imaged Liquid-Based Cytology in Routine Cytology and HPV Cotesting. Am J Clin Pathol 2018;150:385-92. [Crossref] [PubMed]
- Tian Y, Yuan Wu NY, Liou YL, et al. Utility of gene methylation analysis, cytological examination, and HPV-16/18 genotyping in triage of high-risk human papilloma virus-positive women. Oncotarget 2017;8:62274-85. [Crossref] [PubMed]
- Liou YL, Zhang Y, Liu Y, et al. Comparison of HPV genotyping and methylated ZNF582 as triage for women with equivocal liquid-based cytology results. Clin Epigenetics 2015;7:50. [Crossref] [PubMed]
- Liou YL, Zhang TL, Yan T, et al. Combined clinical and genetic testing algorithm for cervical cancer diagnosis. Clin Epigenetics 2016;8:66. [Crossref] [PubMed]
- Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670-5. [Crossref] [PubMed]
- Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt) 2012;21:1031-7. [Crossref] [PubMed]
- Sherman ME, Wang SS, Carreon J, et al. Mortality trends for cervical squamous and adenocarcinoma in the United States. Relation to incidence and survival. Cancer 2005;103:1258-64. [Crossref] [PubMed]
- Pettersson BF, Hellman K, Vaziri R, et al. Cervical cancer in the screening era: who fell victim in spite of successful screening programs? J Gynecol Oncol 2011;22:76-82. [Crossref] [PubMed]
- Visioli CB, Zappa M, Ciatto S, et al. Increasing trends of cervical adenocarcinoma incidence in Central Italy despite Extensive Screening Programme, 1985-2000. Cancer Detect Prev 2004;28:461-4. [Crossref] [PubMed]
- Nguyen TL, Nguyen DC, Nguyen TH, et al. Survey-based cancer mortality in the Lao PDR, 2007-08. Asian Pac J Cancer Prev 2011;12:2495-8. [PubMed]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007;128:683-92. [Crossref] [PubMed]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer 2006;6:107-16. [Crossref] [PubMed]
- Kou Y, Koag MC, Lee S. N7 methylation alters hydrogen-bonding patterns of guanine in duplex DNA. J Am Chem Soc 2015;137:14067-70. [Crossref] [PubMed]
- Lai HC, Lin YW, Huang TH, et al. Identification of novel DNA methylation markers in cervical cancer. Int J Cancer 2008;123:161-7. [Crossref] [PubMed]
- Luan T, Hua Q, Liu X, et al. PAX1 Methylation as a Potential Biomarker to Predict the Progression of Cervical Intraepithelial Neoplasia: A Meta-analysis of Related Studies. Int J Gynecol Cancer 2017;27:1480-8. [Crossref] [PubMed]
- deVos T, Tetzner R, Model F, et al. Circulating methylated SEPT9 DNA in plasma is a biomarker for colorectal cancer. Clin Chem 2009;55:1337-46. [Crossref] [PubMed]
- Kneip C, Schmidt B, Seegebarth A, et al. SHOX2 DNA methylation is a biomarker for the diagnosis of lung cancer in plasma. J Thorac Oncol 2011;6:1632-8. [Crossref] [PubMed]
- Yang HJ, Liu VW, Tsang PC, et al. Comparison of human papillomavirus DNA levels in gynecological cancers: implication for cancer development. Tumour Biol 2003;24:310-6. [Crossref] [PubMed]
- Chen W, Sun H, Molijn A, et al. The Variable Characteristics of Human Papillomavirus in Squamous Cell Carcinoma and Adenocarcinoma of Cervix in China. J Low Genit Tract Dis 2018;22:355-61. [Crossref] [PubMed]
- Molijn A, Jenkins D, Chen W, et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer 2016;138:409-16. [Crossref] [PubMed]
- Guyard A, Boyez A, Pujals A, et al. DNA degrades during storage in formalin-fixed and paraffin-embedded tissue blocks. Virchows Arch 2017;471:491-500. [Crossref] [PubMed]


