
Bronchoscopic ablation techniques in the management of lung cancer
Introduction
Airway involvement in lung cancer is common and often may lead to symptoms of airway obstruction including dyspnea, wheezing, cough, pneumonia, or hemoptysis. There are numerous options available to treat malignant airway obstruction including chemotherapy, radiation, airway stent placement and bronchoscopic ablative techniques. The selection of a specific ablative technique depends primarily upon the clinical urgency to restore airway patency and available technologies. If the patient is presenting with mild or subacute symptoms then multiple modalities can be utilized including those that have a delayed treatment response. If the patient is presenting with more severe symptoms or life-threatening malignant airway obstruction, the patient should be stabilized and then treated with an ablative therapy that will result in a more immediate effect. In addition to computed tomography an initial bronchoscopy is performed to determine what the most appropriate modality may be. Techniques such as laser, electrocautery, argon plasma coagulation (APC), cryoprobe debulking, and airway stenting all result in immediate improvement in airway patency. Other techniques such as photodynamic therapy, cryotherapy, or brachytherapy will have a delayed effect and thus are not suitable for more urgent situations. Balloon dilation can also provide some immediate and transient relief of malignant airway obstruction.
It is important to consider the type of malignancy as well given that in some cases of central airway obstruction with mild air flow limitation other conventional modalities such as external beam radiation therapy (EBRT) or chemotherapy can lead to fairly rapid improvement in airway diameter (i.e., lymphoma, small cell carcinoma). Another main consideration in the selection of an ablation technique is whether the lesion is predominately endobronchial or more submucosal/extrinsic compression. In the latter scenario bronchoscopic options would be more limited to balloon dilation, stent placement, or brachytherapy, with stent placement and balloon dilation providing the most immediate benefit (Table 1).

Full table
Types of bronchoscopy
Flexible bronchoscopy
In some cases of lung cancer airway involvement, flexible bronchoscopic techniques may be adequate to treat the obstruction. The flexible bronchoscope is a thin, flexible scope that is comprised of video or fiberoptic systems that transmit an image from the tip of the instrument to an eyepiece or video camera. At the handle of the scope, a lever adjusts flexion and extension to varying degrees (depending on the manufacturer) at the distal end of the scope; this allows for precise directional movement and targeting of lesions. A working channel within the scope is primarily used for suctioning, as well as insertion of diagnostic and therapeutic instruments into the airway.
An important aspect of the flexible bronchoscope is both the size of the outer diameter as well as the working channel through which therapeutic instruments will be introduced. Flexible bronchoscopes range in size from approximately 3 mm up to 7 mm with significant variability in the inner working channel. Most therapeutic devices require a working channel of at least 2 mm or larger depending on the ablative instrument (1).
Many of the endoluminal interventions, including some of which are performed through rigid bronchoscopy, can be administered through the flexible bronchoscope. The benefit of flexible bronchoscopy is that the equipment is readily available, can be inserted through the nasal passages or oropharyngeal pathway without a secured airway, or through a laryngeal mask airway, endotracheal tube or rigid bronchoscope. Flexible bronchoscopy can be performed under moderate sedation or general anesthesia. Many endoluminal interventions can be administered through a flexible bronchoscope, and this allows for further intervention of smaller distal airways, which may not be accessible by a rigid bronchoscope.
Rigid bronchoscopy
The rigid bronchoscope remains the therapeutic instrument of choice for malignant obstruction of central airways. The large caliber and stiff construction of the rigid bronchoscope allows operators to access and control the airway, as well as accommodate therapeutic tools. The rigid bronchoscope also serves as the means by which patients are ventilated and oxygenated during the procedure. Traditionally, the indications for rigid bronchoscopy include large tissue biopsies, removal of complex foreign bodies, management of massive hemoptysis and delivery of therapeutic interventions for airway obstruction; whether direct or indirect management of endobronchial obstruction or extrinsic compression from malignant or benign etiologies.
There are numerous specific technical advantages to the rigid bronchoscope in comparison to the flexible bronchoscope that need to be emphasized including large volume suction capability, direct airway control, greater accommodation for therapeutic instrumentation, and stent placement. It’s important to remember that the flexible bronchoscope complements the rigid bronchoscope, and may be used as such, to augment ablation of tumors.
The three main components of the rigid bronchoscope are the barrel, the multifunctional head, and the optics with light source (with some variation among manufacturers). The barrel is a hollow metallic tube with a beveled distal tip. The diameter of the barrel varies, ranging from 7.0/6.5 to 16.0/15.0 mm (outer/inner) depending on the manufacturer. The multifunctional head attaches to the proximal portion of the barrel. This piece has ports that accommodate ventilation, procedural instruments and suction simultaneously. Visualization is achieved more commonly by using a telescope and camera or by direct visualization down the barrel with the naked eye and a light source (2).
The approach to lung cancer airway obstruction may employ both rigid and flexible bronchoscopy depending on the clinical scenario. Instruments for use in the airway are designed to pass through the main working channel or barrel of the bronchoscope. Principal accessories include suction catheters, graspers, biopsy forceps, dilators, cautery, stents as well as others. The common feature of instruments designed for the rigid bronchoscope is that they are large and stiffer than those designed for the working channel of the flexible bronchoscope.
Mechanical debulking
Mechanical debulking remains the cornerstone upon which most other bronchoscopic ablation modalities depend. Whether it be primary debulking by mechanical means, or the means by which residual ablated tissue is evacuated from the airway; having expertise with physical debulking instruments is critical for bronchoscopic ablation of tumors.
Flexible mechanical debulking
Utilization of biopsy or debulking forceps through a flexible bronchoscope allow for precise removal of target endobronchial tissue. Using this type of instrument, operators may remove tissue for pathologic examination, or for evacuation of adjunctively ablated tissue. Mechanical flexible forceps exist in many forms and variations exist depending upon the manufacturer. Salient features of these instruments include the size of the forceps (generally range from 1.2 to 2.8 mm or larger), the cup shapes (smooth versus serrated), as well as the configurational design (rat tooth, alligator, needle fenestrated). Complementary use of flexible forceps is commonplace in cases where bronchoscopic tumor ablation is performed. Flexible forceps debulking would only be preferred for smaller airway lesions or in cases where rigid bronchoscopy is not available.
Rigid forceps and related instruments
The rigid bronchoscope itself is a very effective debulking instrument and allows for other rigid instruments to be introduced through it, into the airway. Rigid coring of an endobronchial tumor is an effective means of rapidly removing an endobronchial tumor obstruction; however, extreme caution and expertise is required to ensure appropriate application without damage to adjacent or surrounding structures. By placing the beveled edge of the rigid bronchoscope against the base of the lesion, applying forward pressure while twisting the rigid bronchoscope will bluntly dissect the lesion from the airway wall under direct visualization. While severe bleeding isn’t common, some degree of hemorrhage is expected and operators must have a plan and tools available to achieve hemostasis.
Principal rigid accessories are available for mechanical debulking, and are delivered through the rigid bronchoscope. Rigid forceps of varying sizes and design exist for effective removal of endobronchial tissue and differ depending on the manufacturer. The basic features include length of the instrument, shaft diameter (generally 1.5 to 3 mm), and forceps design (cup, cutting, crocodile jaw, angulated, reverse grasping). Rigid accessory instruments are used either for direct debulking, or for removal of adjunctively ablated tissue.
Mathisen and Grillo previously described the original core-out method with rigid forceps removal; 56 patients underwent this rigid bronchoscopic technique for obstructing airway neoplasms, including 8 patients with distal airway obstructions. Improvement in airway patency was accomplished in 90% of patients. Nineteen major complications were recorded in 11 patients (20%). Although there was no major bleeding seen, there was minor bleeding seen in virtually all patients (3). Another study incorporated this method into their analysis of patients who underwent bronchoscopic ablation, and report lower complication rates, although the data isn’t exclusive to rigid debulking (4).
Microdebrider
The microdebrider is an instrument comprised of three components: a long disposable rotating blade coupled to suction, a handpiece to control directionality and a console, which adjusts the revolution speed. During dissection, tissue is drawn up and into the blade, while debris and blood are rapidly removed from the operating field through suction. The features of this debulking instrument allow for prompt debridement of endobronchial tumors, however operators must be attentive as unintentional damage to normal tissue can occur (5). Initially used through suspension laryngoscopy, a study of 27 patients underwent tumor debulking with the microdebrider and 96% of patients had immediate postoperative stabilization of the airway with a favorable safety profile (6). The effectiveness of this instrument in malignant central airway obstruction was further illustrated in a single center study of 23 patients, who had successful removal of obstructing lesions with interventions lasting 2 to 15 minutes without complications (7). The largest study utilizing this instrument evaluated 51 patients with central airway obstruction, with a mean pretreatment airway obstruction of 71% (49% severe, 39% moderate, 12% mild). After tumor debulking with microdebrider bronchoscopy, the residual mean airway obstruction was 10% (8); the adverse effect rate was reported as 4%, without adverse bleeding events. Other features revealed in this study: the majority of its use was in central airways, the safety with tumors involving the posterior membrane (65% of cases) and its complementary effectiveness with adjunctive bronchoscopic ablative therapies (APC 32%, airway stenting 25%, electrocautery 20%, laser 10%, cryotherapy 10%). Although the safety profile of bronchoscopic microdebrider is optimistic, given the potential for inadvertent tissue destruction, its use should be restricted to centers with expertise in managing central airway obstruction (Figure 1).
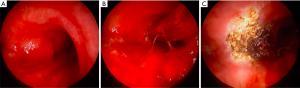
Thermal ablation
Thermal ablation techniques are used in cases of endobronchial disease or in patients with mixed endobronchial/extrinsic lesions. Heat thermal ablation modalities must be used with caution especially in patients with high flow oxygen (those on fraction of inspired oxygen greater than 0.4 secondary to risk of airway fire), and patients with airway stents, or other artificial airway devices in place (endotracheal or tracheostomy tube) as there is a risk of melting or fire. Furthermore, thermal ablation in distal airways may have a higher risk of airway perforation.
Electrocautery
Electrocautery is the use of an electrical probe, knife, biopsy forceps, or snare to conduct monopolar electrical current for heating tissue in contact with or in close proximity to the instrument. Electrical current is conducted by the insulated metal wire probe toward the target tissue. Due to voltage difference between the probe and tissue, electron current density generates heat at the point of contact. Tissue resistance for electrons is high, resulting in vaporization, coagulation or fulguration (9,10).
Thermal destruction of tissue can be used to induce coagulation or resection. Applying the energized instrument directly destroys the target tissue, such that activation creates an immediately visible effect of burning, desiccation and vaporization. Resection can also be accomplished with more polypoid lesions by using a wire snare apparatus looped around the base of the lesion, and energized. Applying traction to the snare while delivering energy excises the lesion at the base, after which the tissue is retrieved by assistance with bronchoscopic suction or graspers. Rigid electrocautery probes are available and used with rigid bronchoscopy while flexible electrodes can be used through the working channel of a flexible bronchoscope. Thus endobronchial electrocautery can be used as a primary modality or often in conjunction with mechanical debulking.
Endobronchial electrocautery is most commonly indicated for the treatment of symptomatic malignant airway obstructions that are not operative candidates, or as adjuncts to other treatment modalities (11-13). In such patients, airway patency is successfully restored in approximately 80% of patients, effectively resulting in symptomatic improvement (11,14). Electrocautery ablation can be applied to bronchogenic carcinomas, endobronchial metastases, bronchial carcinoids (15), and intraluminal microinvasive lung cancer (16,17).
Recently, a device has been developed which incorporates the effects of electrosurgical destruction/resection paired with continuous suction through a soft flexible catheter that can be used with flexible bronchoscopy (CoreCath 2.7S, Medtronic Advanced Energy LLC, Portsmouth, NH, USA) Data on its utility in bronchoscopic tumor ablation is currently limited (18,19).
Endobronchial electrocautery is usually well tolerated, although a number of complications have been described (11-14,20-22). Application of electrocautery near the airway wall has a risk of perforation, potentially leading to pneumothorax and pneumomediastinum. Bleeding can result from tissue destruction, however it generally stops as a result of thermocoagulation, but the risk can be greater depending on the location of the tumor in relation to adjacent vascular structures, as well as tumor type. The use of electricity in the airway has the inherent risk of airway fire, shock and electrical burns if the appropriate precautions are not undertaken. Ventricular fibrillation has occurred when used near the heart, and can cause interference with implanted cardiac pacemakers or defibrillators (23). Generally the complication rates are similar to those encountered using bronchoscopic laser ablation (24).
Laser therapy
Bronchoscopic laser therapy is a thermal ablative technique, which may have cutting and coagulant properties depending on the type of laser, thus making it a useful instrument for treating malignant airway disease. Laser therapy is an immediate-acting, palliative or adjunctive therapy used to relieve central airway obstruction. Different strategies to employ laser techniques depend on appropriate patient selection, properties of the obstructing lesion, and the concomitant use of adjunctive endobronchial therapies.
There are many types of biomedical lasers, each with their own properties. These include Neodymium-yttrium-aluminum-garnet (Nd:YAG) laser, Neodymium-yttrium-aluminum-perovskite (Nd:YAP) laser, carbon dioxide (CO2) laser, argon ion laser, excimer laser, potassium titanyl phosphate (KTP) laser, alexandrite laser, semiconductor laser, pulse dye laser, and holmium YAG laser. The laser that is most commonly used bronchoscopically is the Nd:YAG laser.
Lasers are used for debulking as well as for photocoagulation and devascularization of tumors prior to mechanical debulking. Central airway obstruction from bronchogenic carcinoma is the most common indication for laser resection (25-31). The effects are generally not long lasting and thus are combined with other bronchoscopic therapies, or repeated. Energy is delivered through flexible fibers that are inserted through either a rigid or flexible bronchoscope. Lasers can remove an obstructing airway lesion in two ways: resection in combination with a mechanical modality, or vaporization. In using laser for resection, the laser is directed at the target lesion, devitalizing the lesion through photocoagulation of vascularity. The devitalized tissue is removed through either suction or forceps extraction. Different lasers have characteristic properties including depth of penetration; this can be altered by adjusting the power setting as well as the activation distance from the lesion. Vaporization of tissue is possible as the target tissue contains water. This involves aligning the laser parallel to the bronchial wall and aiming precisely at the lesion. Laser pulses of a few seconds or less are used to vaporize the tissue (Figure 2).
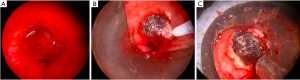
Primary bronchogenic carcinomas are the most common malignancies treated with laser resection, however other tumor types have been treated including carcinoid, adenocystic carcinoma, mucoepidermoid carcinoma, as well as endobronchial metastatic disease from melanoma, colon, kidney and breast cancers (32-35) (Figure 3).
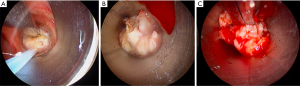
There are no high-quality studies comparing use of laser with other locally ablative therapies, although they all appear to result in similar rates of airway patency and symptom palliation.
Complications can occur with use of laser therapy, but previous reports vary and likely depend on operator experience and tumor characteristics. The overall rates of complications are approximately 2.5% and mortality less than 1% (36,37).
APC
APC is an electrosurgical, noncontact thermal ablation technique that utilizes argon gas for effect, which is used to augment debridement of tissue and to achieve hemostasis. Argon gas is expelled from a probe, which can be introduced through the working channel of a flexible bronchoscope, or rigid bronchoscope, after which voltage electrical current is passed along the probe. Electrical current contacts the gas and it becomes ionized and conducts monopolar electrical current to the nearest tissue (38). Heat produced denatures proteins and evaporates cellular water, which results in tissue destruction and coagulation. The effects of APC are immediate, and are complementary to other modalities in treating tumors within the airway, however concern over long-lasting effects oftentimes leads to requirement of retreatment.
The depth of penetration is approximately 2–3 mm, making APC useful for superficial and flat lesions. Commonly APC is employed to devascularize lesions prior to debulking, but given the superficial effect, APC by itself is not as effective as other modalities in achieving this goal, especially in larger lesions. A desirable effect of APC is that the plasma travels in tangential and linear paths. This improves treatment of lesions around curvatures in the airway and tumor edges, making it very effective at cauterizing hemorrhagic lesions and achieving acute hemostasis.
The data supporting the use of APC for treatment of endobronchial tumors is derived from small observation studies. Given that tumor ablation within the airway is routinely performed in a multimodality approach, it is difficult to attribute all outcomes solely to the effects of APC. Several studies evaluated the effects of APC in malignant central airway obstruction. Reichle et al. showed that approximately two-thirds of patients were able to achieve airway patency with using APC combined with mechanical debulking (39). Studies conducted using APC mostly through flexible bronchoscopy, showed an overall decrease in the degree of airway obstruction, as well as significant symptom improvement (40,41).
The rate of complications vary depending on operator experience, the configuration of individual endobronchial tumors, as well as patient co-morbidities. In a large prospective study of 364 patients, the complication rate was recorded as 3.7% (39). Crosta et al. did not report complications directly related to the use of APC (40). One important consideration when applying APC to the airway is the potential for argon gas entry into the vasculature (through bronchial veins as well as systemic veins); this has been reported to cause argon gas embolism (42,43). More common complications encountered are airway burns/fires, airway perforations, worsening hemorrhage, and melting of stents or other airway tubes.
Cryotherapy
Cryotherapy is the controlled application of extreme cold energy to diseased tissue in which cells are destroyed by intracellular and extracellular cryocrystallization. Application of extreme cold energy causes a cascade of destructive events. Extracellular ice crystal formation causes an efflux of intracellular fluid, which results in cellular dehydration and increased toxicity. Intracellular ice crystal formation causes damage to intracellular organelles such as mitochondria and endoplasmic reticulum. Furthermore, cryotherapy has profound effects on the microcirculation: vasoconstriction, endothelial injury and platelet aggregation. These effects lead to microthrombi formation and subsequent cellular death. Although application of cryotherapy may affect normal tissue, malignant tissue is particularly susceptible given its hypervascularity. The inherent cryosensitivity of a tissue depends mainly on its water content (44).
Application of cryotherapy requires a cryosurgery device (rigid or flexible cryoprobe, spray cryotherapy catheter), a cooling source known as a cryogen (either nitrous oxide or liquid nitrogen), and a bronchoscope through which the therapy can be delivered (either a rigid or flexible bronchoscope) (45,46). The cryoprobe is inserted through the bronchoscope and placed adjacent to the target tissue such that direct contact is made. Repeated freeze-thaw cycles are applied with a freeze time varying depending on the cryogen used. The probe is moved to an untreated area, and repeated freeze-thaw cycles are applied until the entirety of the lesion has been treated. Over the ensuing days to weeks, tissue necrosis occurs followed by sloughing which may be expectorated, or may necessitate bronchoscopic removal.
Cryodebridement or cryorecanalization is a modality of tumor debulking where a cryoprobe is inserted through a bronchoscope, and direct contact to the desired lesion is made. The cryoprobe is activated; the contacted tissue is frozen and becomes adhered to the end of the probe. The probe is retracted along with large fragmented tissue. This technique is helpful for debulking endobronchial tumors in patients unable to tolerate lower fraction of inspired oxygen. Hetzel et al. first described this technique in a prospective trial where they successfully recanalized 83% of patients’ airways by debriding exophytic tumors (47). Schumann et al. later demonstrated its effectiveness in a study of 225 patients, where successful recanalization rate was 91% with an encouraging safety profile; mild bleeding was seen in 4%, moderate bleeding in 8%, with no severe bleeding events encountered (48).
Spray cryotherapy is a non-contact, delayed effect ablative modality that delivers liquid nitrogen through a specialized flexible catheter (TruFreeze system, CSA Medical Inc., USA) which flash freezes 2–3 cm of target area in a rapid and uniform manner (49-51). The depth of freeze correlates to the duration of the application, but generally up to 5 mm after a 5–10 second freeze time. Precautions must be taken to effectively vent the liquid nitrogen from the airway in order to ensure safe application.
Studies evaluating the use of cryotherapy for airway malignancy have shown favorable results. Utilizing cryodebridement, this modality has shown high rates of successful reestablishment of airway patency (47,48). Another prospective study employing cryoablation was able to relieve airway obstruction in 77% of patients (52). Other studies using cryoablation have found similar results with good correlation in symptom improvement (53,54). These effects are likely augmented when using cryotherapy in conjunction with other ablative modalities such as thermal ablation and stenting (48) (Figure 4).
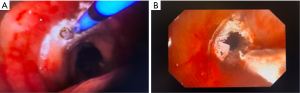
A possible chemo and radiosensitizing effect of cryotherapy had generated significant interest in older studies. Small studies have shown increased accumulation of chemotherapeutic agent within tumors following cryotherapy (55,56). Likewise, in a study of patients with unresectable non-small cell lung cancer (NSCLC), those who underwent cryotherapy prior to external irradiation were found to have a significantly higher rate of local control (57).
A benefit of utilizing cryotherapy is no associated risk of airway fire. However, mild to moderate bleeding can occur in up to 12% of patients, and potentially higher if concurrent debulking techniques are used (48). After applying cryotherapy, delayed effects are expected and include airway edema, as well as mucostasis, which can lead to respiratory distress; operators should be cognizant of this and manage accordingly (58,59). Airway wall ulceration, subsequent perforation, and even death are rare complications, and thus operational familiarity with cryotherapy is of utmost importance.
Although studies report a favorable safety profile with the use of spray cryotherapy, caution must be emphasized when employing this ablative modality (49). In a multi-institutional study, there was a 19% complication rate, which included hypotension (11%), bradycardia (5%), desaturations (6%), pneumothorax (3%), as well as 2 intraoperative deaths (50).
Photodynamic therapy (PDT)
PDT is an ablative technique in which a specific wavelength of light (630 nm laser) is delivered via a flexible catheter (available in multiple lengths) through flexible bronchoscopy in a patient who has been treated with an intravenous photosensitizing agent typically 48 hours prior to the procedure. It is a non-thermal technique and its effects are confined purely to intraluminal disease, with a typical penetration depth in the range of 5–10 mm. The photosensitizing drug is typically a hematoporphyrin derivative and dosimetry is calculated typically to apply 200 Joules per cm (range 50–300 Joules) of airway treated (60,61). In theory the photosensitizing drug is selectively retained for a longer period of time in malignant tissue compared to normal mucosa, thus allowing for selective treatment of abnormal tissue at 48 hours, and possibly an additional treatment over the next 3–5 days. Activation of the photosensitizing drug results in superoxide and oxygen radical formation leading to apoptosis and cell death. Due to tissue sloughing and necrosis a second bronchoscopy is often necessary to remove debris.
Patients with symptomatic malignant airway obstruction may be candidates for PDT however, like brachytherapy the effects are delayed thus patients with severe symptoms or life-threatening obstruction should be considered for an alternative more immediate therapy. For palliative intent lesions must be mostly endobronchial with significant airway obstruction. Although rarely done, if utilized for curative intent the lesion must be short without deep penetration through the airway wall.
Much of the early PDT literature investigated its effectiveness in early central airway lung cancer. In one of the largest series, Kato et al. treated 283 lesions, 95 of which were early stage lesions and demonstrated a complete response in 83% of the early stage lesions (62). Multiple palliative studies have demonstrated significant improvement in airway patency and symptoms in patients with central airway obstruction from advanced lung cancer (63,64).
Patients with predominately extrinsic malignant airway obstruction, airway fistula, and those with long lesions or lesions adjacent to larger blood vessels should not undergo PDT. Also those with more clinically urgent needs for treatment of symptoms should not be treated with PDT due to the delayed effect. Major complications include airway obstruction 24 to 48 hours after treatment from sloughing tissue or edema, hemoptysis, and photosensitivity. The resultant skin sensitivity is significant and patients must take protective measures up to six weeks following injection.
Endobronchial brachytherapy
Brachytherapy is the process by which a source of radiotherapy is placed inside or adjacent to the treatment target, resulting in a highly localized radiation dose with excellent sparing of surrounding normal tissue. In the lung, brachytherapy can be delivered through an endobronchial or intraluminal technique, interstitial brachytherapy in which the radiation source is typically placed directly in the tissue at the time of surgery, or through image guided brachytherapy delivered through a catheter technique (65). First described in 1922, the vast majority of cases of endobronchial brachytherapy (EBBT) are performed for palliation of endobronchial lung cancer or other metastatic malignancies and typically utilize 192Ir (66). In rare cases it may also be performed as definitive therapy for carcinoma in situ, benign conditions, and in conjunction with external beam radiotherapy. Patients previously treated with EBRT who are not candidates for further EBRT may also be treated with EBBT (67). In general the utilization of EBBT has declined due to further development and growth of alternative bronchoscopic ablative techniques such as electrocautery, cryotherapy, APC, and laser.
Types of EBBT include low-dose rate (LDR), high-dose rate (HDR), and pulse dose rate (PDR). LDR delivers a lower dose of typically 1–2 Gy/hour given over multiple days. This type of therapy is labor intensive requiring an airway catheter to be left in place continuously as well as the need for significant radiation precautions in the inpatient setting. HDR is delivered more efficiently with higher energy radiation often of 10–12 Gy/hour but usually delivered over one or more treatment procedures. Many different treatment regimens are used but commonly patients will undergo 2–4 fractions. PDR brachytherapy combines aspects of LDR and HDR delivering the radiation in a series of short exposures each hour, however it is not frequently used and not preferred for endobronchial disease (67).
Patient selection is based on the typical symptoms of malignant airway obstruction including dyspnea, cough, lobar collapse, or post-obstructive pneumonia. These patients often have not responded to or are not candidates for standard chemotherapy or EBRT. The lesion must be accessible to bronchoscopy including placement of a small-bore catheter in the airway. Tumors in the airway that show significant vascular involvement or ulceration should be considered for other options. It is important to note that patients with acute or severe symptoms of airway obstruction should have an alternative ablative technique as first-line treatment due to the delayed effect of EBBT on restoring airway patency. As a general rule EBBT is typically not selected or recommended as a first-line therapy for obstructing endobronchial malignancy (67). EBBT can be performed in the vicinity of metal airway stents but data from esophageal metal stents show that mucosal doses can be increased substantially in the immediate adjacent tissue (68).
The procedure is typically performed with flexible bronchoscopy which can be done under moderate sedation or general anesthesia. If performed with moderate sedation the catheter is typically placed intranasally. Flexible bronchoscopy is used to place a guidewire into the correct airway, then removed and the brachytherapy catheter is advanced over the guidewire under bronchoscopic guidance. The distal end of the catheter is typically placed approximately 2 cm beyond the distal edge of the target lesion. If a transnasal approach is utilized the catheter is marked and taped on the nostril at the exit site and the position of the brachytherapy catheter and measurements must be carefully documented to assist the radiation team in treatment delivery. It is important for the bronchoscopist to take photographs as well if possible during a prior procedure to assist in radiation therapy planning. CT scanning is also recommended as an essential part of planning with three-dimensional target definition and to help determine if there is significant extra bronchial tumor which would affect the decision to perform EBBT. In the latest American Brachytherapy Society Guidelines, CT scanning is also recommended after the brachytherapy catheter has been placed to accurately assess the position of the catheter and its proximity to the airway wall, as it may not sit perfectly centered in the lumen of the bronchus (67). Once positioned correctly the radiation source is remotely loaded into the catheter at the correct length, and then the catheter is removed immediately following administration (in HDR).
A Cochran meta-analysis in 2012 reviewed 14 randomized clinical trials involving EBBT both as isolated therapy or in combination with other modalities. There was no clear survival advantage with fewer vs. multiple fractions of EBBT or when added to EBRT or compared to Nd:YAG laser (69). A large retrospective study of 648 patients showed no difference in efficacy or survival in groups treated with 1 fraction versus multiple fractions (70). Another larger randomized trial of 142 patients showed improved local tumor response in 2 fractions verses 4 fractions with similar overall survival, but a trend towards reduced fatal hemoptysis with fewer fractions (71).
Patients with endobronchial ulceration, fistula formation, or severe airway tumor obstruction are typically not recommended for EBBT. Complications include radiation bronchitis, bronchial stenosis, and massive hemoptysis. Fatal hemoptysis has ranged from 7% to as high as 22% in published trials (70). Patients may require monitoring for transient worsening airway obstruction from sloughing tissue or edema, and may require repeat bronchoscopy in the first few days following treatment.
Conclusions
Airway obstruction is a common manifestation of lung cancer and often may lead to symptoms of airway obstruction including dyspnea, wheezing, cough, pneumonia, or hemoptysis. There are numerous options available to treat malignant airway obstruction including chemotherapy, radiation, airway stent placement and bronchoscopic ablative techniques. Bronchoscopic ablative techniques are very effective but diverse in nature, thus selecting specific ablative techniques depends primarily upon the clinical urgency to restore airway patency and available technologies. Techniques such as laser, electrocautery, APC, cryoprobe debulking, and airway stenting all result in immediate improvement in airway patency. Other techniques such as photodynamic therapy, cryotherapy, or brachytherapy will have a delayed effect and thus are not suitable for more urgent situations. The second main consideration in selection of an ablation technique is whether the lesion is predominately endobronchial or more submucosal/extrinsic compression. In the latter scenario bronchoscopic options would be more limited to balloon dilation, stent placement, or brachytherapy, with stent placement and balloon dilation providing the most immediate benefit. It is also important to consider the type of malignancy as well given that in some cases of malignant central airway obstruction the obstruction may respond quickly to conventional modalities such as EBRT or chemotherapy. In summary, there are numerous effective techniques available to offer these patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Garland R. The bronchoscope: what is available, determining selection, and how to properly care for the instrument. In: Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. New York, NY: Springer, 2016:27-36.
- Gorden JA. Rigid bronchoscopy. In: Ernst A, Herth FJ. editors. Principles and Practice of Interventional Pulmonology. New York, NY: Springer, 2016:285-95.
- Mathisen DJ, Grillo HC. Endoscopic Relief of Malignant Airway Obstruction. Ann Thorac Surg 1989;48:469-73. [Crossref] [PubMed]
- Stephens KE Jr, Wood DE. Bronchoscopic management of central airway obstruction. J Thorac Cardiovasc Surg 2000;119:289-96. [Crossref] [PubMed]
- Kühnel T, Hosemann W, Rothammer R. Evaluation of powered instrumentation in out-patient revisional sinus surgery. Rhinology 2001;39:215-9. [PubMed]
- Simoni P, Peters GE, Magnuson JS, et al. Use of the endoscopic microdebrider in the management of airway obstruction from laryngotracheal carcinoma. Ann Otol Rhinol Laryngol 2003;112:11-3. [Crossref] [PubMed]
- Lunn W, Garland R, Ashiku S, et al. Microdebrider Bronchoscopy : A New Tool for the Interventional Bronchoscopist. Ann Thorac Surg 2005;80:1485-8. [Crossref] [PubMed]
- Casal RF, Iribarren J, Eapen G, et al. Safety and effectiveness of microdebrider bronchoscopy for the management of central airway obstruction. Respirology 2013;18:1011-5. [Crossref] [PubMed]
- Sutedja TG. Electrosurgery. In: Ernst A, Herth FJ. editors. Principles and Practice of Interventional Pulmonology. New York, NY: Springer, 2016:337-41.
- Barlow DE. Endoscopic applications of electrosurgery: a review of basic principles. Gastrointest Endosc 1982;28:73-6. [Crossref] [PubMed]
- Sutedja G, van Kralingen K, Schramel FM, et al. Fibreoptic bronchoscopic electrosurgery under local anaesthesia for rapid palliation in patients with central airway malignancies: a preliminary report. Thorax 1994;49:1243-6. [Crossref] [PubMed]
- Baldeyrou P, Girard P, Grunenwald D. High-frequency thermocoagulation of tumors of the respiratory tract: Results of an initial study with bronchofiberscope. J Bronchol 1996;3:243. [Crossref]
- Pedersen U, Kristensen S, Illum P. Palliative resection with high-frequency cutting loop in malignant tracheobronchial diseases. J Bronchol 1994;1:23-5. [Crossref]
- Wahidi MM, Unroe MA, Adlakha N, et al. The use of electrocautery as the primary ablation modality for malignant and benign airway obstruction. J Thorac Oncol 2011;6:1516-20. [Crossref] [PubMed]
- Sutedja G, Schramel FM, Smit HJ, et al. A prospective study of bronchoscopic electrocautery (BE) in patients with intra luminal typical bronchial carcinoid (ITBC). Eur Respir J 1996;23:258S.
- van Boxem TJ, Venmans BJ, Schramel FM, et al. Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 1998;11:169-72. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Bronchoscopic treatment of patients with intraluminal microinvasive radiographically occult lung cancer not eligible for surgical resection: a follow-up study. Lung Cancer 2003;39:49-53. [Crossref] [PubMed]
- Technology corner. A novel electrocautery instrument for central airway obstruction. Available online: https://www.wabip.com/downloads/newsletter/web/2019-01
- Mahajan AK, Herdina KA, Howk KA, et al. Performance of a Novel Electrosurgical Device for Cutting and Coagulation of Central Airway Obstructions. Am J Respir Crit Care Med 2018;197:A7329.
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1985;87:712-4. [Crossref] [PubMed]
- Gerasin VA, Shafirovsky BB. Endobronchial electrosurgery. Chest 1988;93:270-4. [Crossref] [PubMed]
- Hooper RG, Jackson FN. Endobronchial electrocautery. Chest 1988;94:595-8. [Crossref] [PubMed]
- Caramella JP, Dodinot B. Cardiac pacemaker deprogramming by electrocautery. An update. Ann Fr Anesth Reanim 1989;8:290. [Crossref] [PubMed]
- Kvale PA, Selecky PA, Prakash UB, et al. Palliative care in lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:368S-403S.
- Ernst A, Silvestri GA, Johnstone D, et al. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest 2003;123:1693-717. [Crossref] [PubMed]
- Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73. [PubMed]
- Daddi G, Puma F, Avenia N, et al. Resection with curative intent after endoscopic treatment of airway obstruction. Ann Thorac Surg 1998;65:203-7. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. Summary of the British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68:786-7. [Crossref] [PubMed]
- Hermes A, Heigener D, Gatzemeier U, et al. Efficacy and safety of bronchoscopic laser therapy in patients with tracheal and bronchial obstruction: a retrospective single institution report. Clin Respir J 2012;6:67-71. [Crossref] [PubMed]
- Simoff MJ, Lally B, Slade MG, et al. Symptom management in patients with lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e455S-97S.
- Colt HG, Harrell JH. Therapeutic rigid bronchoscopy allows level of care changes in patients with acute respiratory failure from central airways obstruction. Chest 1997;112:202-6. [Crossref] [PubMed]
- Neyman K, Sundset A, Naalsund A, et al. Endoscopic treatment of bronchial carcinoids in comparison to surgical resection: a retrospective study. J Bronchology Interv Pulmonol 2012;19:29-34. [Crossref] [PubMed]
- Mehta AC, Golish JA, Ahmad M, et al. Palliative treatment of malignant airway obstruction by Nd-YAG laser. Cleve Clin Q 1985;52:513-24. [Crossref] [PubMed]
- Carlin BW, Harrell JH 2nd, Olson LK, et al. Endobronchial metastases due to colorectal carcinoma. Chest 1989;96:1110-4. [Crossref] [PubMed]
- Cavaliere F, Dumon JF. Laser bronchoscopy. In: Bollinger CT, Mathur PN. editors. Interventional bronchoscopy. Basel: Karger AG, 2000:108.
- Squiers JJ, Teeter WA, Hoopman JE, et al. Holmium:YAG laser bronchoscopy ablation of benign and malignant airway obstructions: an 8-year experience. Lasers Med Sci 2014;29:1437-43. [Crossref] [PubMed]
- Beamis JF Jr, Vergos K, Rebeiz EE, et al. Endoscopic laser therapy for obstructing tracheobronchial lesions. Ann Otol Rhinol Laryngol 1991;100:413-9. [Crossref] [PubMed]
- Platt RC. Argon plasma electrosurgical coagulation. Biomed Sci Instrum 1997;34:332-7. [PubMed]
- Reichle G, Freitag L, Kullmann HJ, et al. Argon plasma coagulation in bronchology: a new method--alternative or complementary? Pneumologie 2000;54:508-16. [Crossref] [PubMed]
- Crosta C, Spaggiari L, De Stefano A, et al. Endoscopic argon plasma coagulation for palliative treatment of malignant airway obstructions: early results in 47 cases. Lung Cancer 2001;33:75-80. [Crossref] [PubMed]
- Morice RC, Ece T, Ece F, et al. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest 2001;119:781-7. [Crossref] [PubMed]
- Reddy C, Majid A, Michaud G, et al. Gas embolism following bronchoscopic argon plasma coagulation: a case series. Chest 2008;134:1066-9. [Crossref] [PubMed]
- Shaw Y, Yoneda KY, Chan AL. Cerebral gas embolism from bronchoscopic argon plasma coagulation: a case report. Respiration 2012;83:267-70. [Crossref] [PubMed]
- Ramez S. Cryotherapy and cryodebridement. In: Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. New York, NY: Springer, 2016:343-50.
- Homasson JP, Bell NJ. Cryotherapy in chest medicine. Paris: Springer Verlag, 1992.
- Noppen M, Meysman M, Van Herreweghe R, et al. Bronchoscopic cryotherapy: preliminary experience. Acta Clin Belg 2001;56:73-7. [Crossref] [PubMed]
- Hetzel M, Hetzel J, Schumann C, et al. Cryorecanalization: a new approach for the immediate management of acute airway obstruction. J Thorac Cardiovasc Surg 2004;127:1427-31. [Crossref] [PubMed]
- Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010;139:997-1000. [Crossref] [PubMed]
- Krimsky WS, Broussard JN, Sarkar SA, et al. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg 2010;139:781-2. [Crossref] [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203. [Crossref] [PubMed]
- Browning R, Parrish S, Sarkar S, et al. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis 2013;5:E103-6. [PubMed]
- Walsh DA, Maiwand MO, Nath AR, et al. Bronchoscopic cryotherapy for advanced bronchial carcinoma. Thorax 1990;45:509-13. [Crossref] [PubMed]
- Maiwand MO, Homasson JP. Cryotherapy for tracheobronchial disorders. Clin Chest Med 1995;16:427-43. [PubMed]
- Mathur PN, Wolf KM, Busk MF, et al. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest 1996;110:718-23. [Crossref] [PubMed]
- Ikekawa S, Ishihara K, Tanaka S, et al. Basic studies of cryochemotherapy in a murine tumor system. Cryobiology 1985;22:477-83. [Crossref] [PubMed]
- Homasson JP, Pecking A, Roden S, et al. Tumor fixation of bleomycin labeled with 57 cobalt before and after cryotherapy of bronchial carcinoma. Cryobiology 1992;29:543-8. [Crossref] [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40. [Crossref] [PubMed]
- Niu L, Xu K, Mu F. Review Article Cryosurgery for lung cancer. J Thorac Dis 2012;4:408-19. [PubMed]
- Maiwand MO, Asimakopoulos G. Cryosurgery for Lung Cancer: Clinical Results and Technical Aspects. Technol Cancer Res Treat 2004;3:143-50. [Crossref] [PubMed]
- Reddy C, Photodynamic therapy. In: Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. New York: Springer, 2013:377-85.
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Kato H, Okunaka T, Shimatani H. Photodynamic therapy for early stage bronchogenic carcinoma. J Clin Laser Med Surg 1996;14:235-8. [Crossref] [PubMed]
- Minnich DJ, Bryant AS, Dooley A, et al. Photodynamic laser therapy for lesions in the airway. Ann Thorac Surg 2010;89:1744-8. [Crossref] [PubMed]
- Moghissi K, Dixon K, Stringer M, et al. The place of bronchoscopic photodynamic therapy in advanced unresectable lung cancer: experience of 100 cases. Eur J Cardiothorac Surg 1999;15:1-6. [Crossref] [PubMed]
- Tabba M. Brachytherapy. In: Ernst A, Herth FJ. Principles and Practice of Interventional Pulmonology. New York: Springer, 2013:367-76.
- Yankauer S. Two cases of lung tumor treated bronchoscopically. NY Med J 1922;21:741.
- Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 2016;15:1-11. [Crossref] [PubMed]
- Li XA, Chibani O, Greenwald B, et al. Radiotherapy dose perturbation of metallic esophageal stents. Int J Radiat Oncol Biol Phys 2002;54:1276e1285.
- Reveiz L, Rueda JR, Cardona AF. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev 2012;12:CD004284. [PubMed]
- Skowronek J, Kubaszewska M, Kanikowski M, et al. HDR endobronchial brachytherapy (HDRBT) in the management of advanced lung cancer-comparison of two different dose schedules. Radiother Oncol 2009;93:436e440.
- Niemoeller OM, Pollinger B, Niyazi M, et al. Mature results of a randomized trial comparing two fractionation schedules of high dose rate endoluminal brachytherapy for the treatment of endobronchial tumors. Radiat Oncol 2013;8:8. [Crossref] [PubMed]


