
N-terminal pro-B-type natriuretic peptide for predicting fluid challenge in patients with septic shock
Introduction
Fluid resuscitation is an important component in the management of septic shock (1,2). However, only 40–50% of patients with unstable haemodynamics can benefit from fluid therapy (3). On the other hand, fluid overload is associated with increased mortality in critically ill patients (4). Therefore, much attention is being paid to the assessment of fluid responsiveness, i.e., the response of cardiac output (CO) or stroke volume (SV) to fluid challenge (FC). Although various methods have been evaluated independently as predictors of fluid responsiveness (5), FC remains the most commonly used in clinical practice (6).
The N-terminal pro-B-type natriuretic peptide (NT-proBNP), a biologically inactive cleavage product of the prohormone BNP, is synthesized and secreted from ventricular cardiomyocytes into the blood in response to increased myocardial stretch (7,8). Since both kinds of BNPs (this term represents either BNP or NT-proBNP throughout the remainder of the paper) are considerably higher in patients with acute destabilized heart failure, testing for BNP levels has been recognized and widely used for routine clinical diagnostics and management in the context of heart failure (9). For this reason, when facing septic shock patients with high plasma BNP levels, intensivists may also hesitate to give FC, fearing overloaded cardiac filling and thus the absence of fluid responsiveness state. Indeed, elevated BNP levels are frequently measured in patients with septic shock and are considered as an indicator of sepsis-induced myocardial dysfunction (10,11). Recently, several studies had examined the prognostic values of BNP levels and their potential role in guiding fluid therapy in septic patients (12,13); however, whether BNP levels have predictive value for fluid responsiveness in septic shock patients remains questionable.
Several studies on the topic have been published with conflicting results (14-17). The inclusion of different patient populations and relatively small numbers of patients enrolled might compromise the reliability of these results. In addition, differences related to age, sex, renal function or other treatment modalities have been identified to influence plasma BNP values (14-18), thus adding to this discrepancy.
Therefore, to clarify these issues, we performed this retrospective observational study to examine whether the knowledge of plasma NT-proBNP concentration could predict fluid responsiveness in septic shock patients. Moreover, we further explored the potential confounding effects on the diagnostic accuracy of NT-proBNP in predicting fluid responsiveness.
Methods
Study design
This was a respective observation study using data from a registry clinical trial focusing on the physiological changes defining fluid responsiveness in patients with septic shock. The clinical trial was registered at ClinicalTrial.gov (NCT01941472). Data of some patients have been reported in previous articles (19). The study protocol was approved by the Ethical Committee of Peking Union Medical College Hospital. Informed consent was waived given the observational nature of the study.
Patients
Adult patients diagnosed with septic shock who required CO monitoring during their ICU stay from September 2015 to July 2018 were studied. Septic shock was defined according to international criteria (20). All these patients received initial fluid resuscitation [defined as 30 mL/kg of intravenous fluid administered or a central venous pressure (CVP) of no less than 8 mmHg was achieved] (2,21). According to the protocol, patients with evidence of cardiac dysfunction (i.e., acute pulmonary oedema, acute coronary syndrome or cardiogenic shock), age less than 18 years, known allergy to colloid fluids, pregnancy, recent participation in another biomedical study, a requirement for blood transfusion, and a life expectancy less than 24 hours were excluded.
Haemodynamic monitoring
Arterial blood pressure was monitored from an arterial line placed in a radial artery or femoral artery. CVP was measured with a venous central catheter (CV-15854; Arrow International, Reading, PA, USA) inserted into the internal jugular vein. CVP and blood pressure were measured with a transducer zeroed at the level of the midaxillary line. Cardiac index (CI) was calculated by the continuous thermodilution technique in patients equipped with a pulmonary artery catheter (PAC) device (Swan Ganz CCOmbo, Edwards Lifesciences, Irvine, CA, USA) or a pulse-induced contour CO (PiCCO) device (Pulsion Medical Systems, Munich, Germany). Adequate position of the PAC was confirmed by haemodynamic waveform analysis and chest X-ray. The CVP and all PAC-derived haemodynamic variables were measured at end-expiration. All the above catheters connected to the pressure transducer and the IntelliVue Patient Monitor MP70 (Philips Medical System, Boeblingen, Germany).
FC
The reasons for FC included sepsis-induced hypotension [systolic blood pressure (SBP) <90 mmHg or mean arterial pressure (MAP) <65 mmHg or the need for vasopressor infusion] and the presence of tissue hypoperfusion (including but not limited to oliguria, skin mottling, cool peripheries, altered mental status, hyperlactatemia, and an increased requirement for catecholamines). For FC, 500 mL of 4% gelatine [Gelofusine; B. Braun Medical (Suzhou) Company Limited, Suzhou, China] or 0.9% normal saline were administered over 5–10 min using a bag pressurized to 300 mmHg. The choice of infusion fluid and the decision to stop the FC out of safety concerns of the patients were at the discretion of the treating physicians. An increased in CI greater than or equal to 10% after FC was defined as fluid responsiveness.
Data collection
Data on demographics, type of infusion fluid, APACHE II score, underlying diseases, the number of organ dysfunctions and clinical data concerning therapies (e.g., respiratory support, renal replacement therapy, inotropic agents) were collected for all included patients. Plasma NT-proBNP levels and creatinine concentrations were obtained before FC. The detectable range of NT-proBNP concentration is 0–35,000 pg/mL. Readings >35,000 pg/mL were recorded as 35,000 pg/mL. We used the method of Cockcroft and Gault to estimate glomerular filtration rate (eGFR) (22), and an eGFR <60 mL/min is defined as impaired renal function (23). Haemodynamic variables for analysis, including MAP, heart rate (HR), CI, CVP, pulmonary arterial wedge pressure (PAWP), and systemic vascular resistance index (SVRI), were obtained before and within 10 min after FC. Absolute changes (expressed in ∆) and relative changes (also expressed in %) in these variables after FC were also calculated (24).
Statistical analysis
The variables were expressed as the mean value ± SD or the median value along with 25–75% interquartile range (IQR) as appropriate. The D’Agostino-Pearson omnibus normality test was used to test for normal distribution before further analysis. Continuous data were analysed using Student’s t-test or paired t-test. Regarding categorical data, Chi-Square test, Fisher’s exact tests or Mann-Whitney test were used. Correlation between variables was assessed using Pearson’s correlation. Because NT-proBNP data were not normally distributed, log NT-proBNP were used in the correlations. Receiver operating characteristic (ROC) curves was established for NT-proBNP levels and changes in MAP, HR and CVP as indicators of fluid responsiveness. Testing the potential confounding factors of the discriminative performance of NT-proBNP in fluid responsiveness, we conducted subgroup analyses as follows: (I) NT-proBNP levels (<25th, 25th–75th, >75th percentiles); (II) patient age (ages <50, 50–75, and >75 years); (III) eGFR level (<60 or ≥60 mL/min); (IV) type of fluid used (4% gelatine or 0.9% normal saline); (V) sex; and (VI) haemodynamic monitor (PAC or PiCCO). Statistical analyses were performed and plots were generated using MedCalc Statistical Software version 15.6.1 (Ostend, Belgium). A P value <0.05 was considered to indicate statistical significance.
Results
Patient characteristics
A total of 79 patients were included. Demographic data are shown in Table 1. Patients had a mean age of 59 years and a mean APACHE II score of 25 at enrolment. Fifty-five patients (69%) were classified as responders. The most common site of infection was the lung, with an incidence of 67%. There were no significant differences between the groups in terms of fluid for FC with 4% gelatine being the most commonly used. The median FC time was 6.4 min (5.4–8.5 min). Thirty-four patients (43%) died during ICU stay.
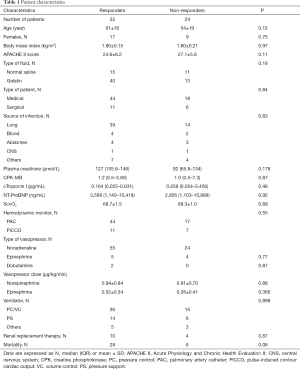
Full table
Haemodynamic characteristics
No complications were observed during FC. Haemodynamic variables before and after FC are presented in Table 2. All physiologic parameters were comparable between the two groups before FC. After FC, we observed a significant increase in CVP, HR, and PAWP in both groups, whereas MAP, SBP, and diastolic blood pressure (DBP) increased significantly in responders but not in non-responders (P<0.05). For all predefined haemodynamic variables, changes in values after FC showed very poor discriminative performance (Table 3).

Full table

Full table
NT-proBNP
A wide range of baseline NT-proBNP levels was observed (48 to >35,000 pg/mL) with a median log NT-proBNP of 3,019 (1,114–10,419) pg/mL for all patients. There was no difference in median NT-proBNP levels between responders and non-responders (log 3,568 versus log 2,856 pg/mL; P=0.915). No significant correlation was observed between NT-proBNP and %CI (r=−0.104, P=0.361) following FC for all patients or when the responders exclusively were considered (r=−0.026, P=0.851). This result remained unchanged when ∆CI and NT-proBNP (r=−0.159, P=0.161) or %SV and NT-proBNP (r=−0.12, P=0.137) were also evaluated. In addition, no correction was observed between NT-proBNP and vasopressor requirement (r=−0.072, P=0.538).
Overall, the baseline NT-proBNP levels could not identify responders to FC with an AUC of 0.508 (95% CI, 0.369–0.647). In the subgroup of patients with different NT-proBNP levels (<25th, 25th–75th, >75th percentiles), NT-proBNP was also not a good predictor of %CI after FC with AUC values of 0.650, 0.50, and 0.523, respectively. Among those patients <50, 50–75, and >75 years of age, NT-proBNP had AUC values of 0.559, 0.513, and 0.667, respectively, suggesting no further advantage in diagnostic performance by age stratification. Similarly, NT-ProBNP could not differentiate CO responders and non-responders when patients who used 4% gelatine (AUC 0.563) or normal saline (AUC 0.60) were considered. The NT-proBNP levels were approximately three-fold higher in patients with an eGFR <60 mL/min versus an eGFR ≥60 mL/min. However, the NT-proBNP levels could not accurately predict fluid responsiveness in both groups. In addition, poor diagnostic performance of NT-proBNP levels was also confirmed when gender (female: AUC 0.601; male: AUC 0.554) or haemodynamic monitor (PAC: AUC 0.553; PiCCO: AUC 0.661) were considered (Table 4).
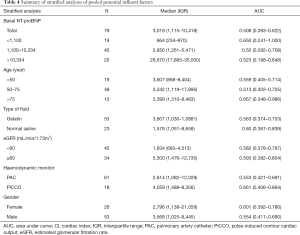
Full table
Discussion
Our study showed that high NT-proBNP levels were widely found in patients with septic shock. There was no significant correlation between NT-proBNP level and CI change after FC for all the patients or when only the responders were considered. Similarly, the knowledge of NT-proBNP level could not accurately predict fluid responsiveness in such patient populations. This result was confirmed by further subgroup analyses.
Despite initial fluid resuscitation, the studied population remained severely shocked. These patients had a mean arterial lactate level of 4.7–5.1 mmol/L and a mean ScvO2 of 68% and were supported by a high dose of vasopressors (e.g., norepinephrine 0.93 µg/kg/min) at inclusion. We found the median NT-proBNP level was considerably elevated (3,019 pg/mL) in our study cohort, which agreed with some previous literature (14,25).
Several studies have evaluated the diagnostic value of NT-proBNP or BNP levels in predicting fluid responsiveness (14-16). In contrast to our findings, Hartemink et al. reported that baseline NT-proBNP levels inversely correlated to the increase in CI after FC (r=−0.57, P=0.032), and a high NT-proBNP (>3,467 pg/mL) was a better predictor of fluid nonresponsiveness in sepsis (AUC =0.75, P=0.049) (14). However, only 18 septic patients were included in that study, whereas we included 79 patients focusing exclusively on septic shock. Therefore, their patients had relatively lower APACHE II scores compared with that of our study (12 vs. 26), which might contribute to the difference. Moreover, patients included in that study (14) received intravenous fluids for an average of 1,500 mL during FC, a larger volume than that of currently recommended (500 mL) (24). Remarkably, fluid loading was performed with a maximum of 200 mL of infused volume per 10 min in that study (14), leading to a longer FC time (approximately 90 min), which might affect the proportion of volume infused remaining in the intravascular compartment at the end of FC, especially in septic patients with vascular hyperpermeability. In another study, Muller et al. suggested that BNP did not accurately predict fluid responsiveness in patients with acute circulatory failure (15). However, in that study, 24% (8/33) of the patient population suffered from causes other than sepsis and reported a lower baseline BNP concentration value in responders. The latter might explain why most non-septic shock patients (7/8, 88%) were responders because NT-proBNP plasma levels have been repeatedly shown to be higher in patients with sepsis than those without sepsis (14,26,27). The volume of FC used in that study was 250 or 500 mL, and different doses of fluids used for FC can modify the proportions of responders in study populations (28). In addition, ScvO2 <70% was defined as a criterion for FC in that study (15). However, the presence of ScvO2 >70% does not exclude fluid responsiveness in critically ill septic patients (29). In a study of 23 septic and septic shock patients, Pirracchio et al. also reported no correlation between baseline BNP levels and fluid responsiveness (16). In addition, nine of the 11 patients with baseline BNP >1,000 pg/mL were fluid responders. Similar findings were also observed in our study, and 53% (32/60) of patients with NT-proBNP >1,100 ng/mL showed a response to FC.
Similar to static markers of cardiac preload, such as CVP and PAWP (30), NT-proBNP shows poor prediction of fluid responsiveness in the present study. This finding can be explained by several factors. First, elevated BNP levels are not exclusively determined by severely impaired myocardial function due to sepsis (10,11) but may be due to several potential confounders, including stimulation of lipopolysaccharide (31), sepsis-induced biventricular dilatation (14,29), over-secretion of proinflammatory cytokines (27), vasoactive and inotropic drugs (32), renal failure (18), and sepsis-associated acute lung injury (33). Therefore, the lack of specificity of NT-proBNP as a marker of cardiac failure in the presence of septic shock makes it impossible to accurately predict fluid responsiveness. Second, NT-proBNP may not be a clinically useful non-invasive marker of cardiac filling pressures in critically ill patients. In a study of 49 septic shock patients, Tung et al. compared BNP values and parameters obtained by right heart catheterization and found no correlation between BNP levels and PCWP (34). Similar findings were also found in other studies (25,35) or the current study. Third, sepsis-induced reversible myocardial dysfunction may not necessarily lead to decreased volume per stroke. Among 34 patients with severe sepsis and septic shock, Charpentier et al. reported that elevated BNP levels were also observed in patients with a fractional area contraction >50% (10). In addition, an increase in end-diastolic volume due to adequate fluid resuscitation or high doses of catecholamine infusions can compensate for this reduction. Finally, in addition to acting as preload, fluid administered during FC can decrease afterload by fluid-induced haemodilution. In the study by Monge García et al., the authors performed FC in 81 septic shock patients and evaluated the SV/CO using Doppler transoesophageal echocardiography and found a 10% decrease in the SVR following FC in responders (36). This phenomenon was also observed in our study. Therefore, if afterload is decreased by haemodilution due to fluid administration, the CO may increase despite the low left ventricular function.
Another important finding is that stratified analysis of several influencing factors did not significantly improve the predictive accuracy of NT-proBNP, which supported the robustness of our main outcome. Noteworthy, some subgroups (i.e., age, glomerular filtration) are grouped on the basis of data from patients with heart failure (37), which is due to the current lack of guidance on how NT-proBNP values are adjusted for these confounders in septic shock. Thus, whether these values are equally applicable to septic shock requires further research.
Our study acknowledges some limitations. First, NT-proBNP level measurement after FC was not performed; thus, the effect of FC on NT-proBNP levels was unavailable. However, previous studies have shown that NT-proBNP levels remain unchanged after FC (14). Notably, a delayed increase in NT-proBNP after FC has been reported in healthy volunteers or patients with heart failure (38,39). However, regarding septic patients, the appropriate NT-proBNP monitoring time after FC is unclear. Second, the included patients were administered 500 mL of 4% gelatine or 0.9% normal saline during FC. Theoretically, up to two to three times as much crystalloid as colloid may be required to maintain intravascular volume given differences in intravascular half-life. However, we assessed fluid responsiveness immediately after an average of 6 min of FC compared with 30 min in previous studies (14,15). Therefore, a large proportion of the volume might still remain in the intravascular compartment in such a short time, causing less difference between both liquids in our study. Third, we did not investigate the effects of other potential confounders, such as dose of vasopressor, underlying disease, and fluid balance, all of which may play a role in NT-proBNP concentration.
Conclusions
High NT-proBNP levels were commonly found in patients with septic shock. However, we should not use baseline NT-proBNP levels as the only indicator to withhold FC but rather as a precaution to fluid resuscitation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Ethical Committee of Peking Union Medical College Hospital (No. ZS1085). Informed consent was waived given the observational nature of the study.
References
- Vincent JL, Gerlach H. Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit Care Med 2004;32:S451-4. [Crossref] [PubMed]
- Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med 2010;36:222-31. [Crossref] [PubMed]
- Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest 2002;121:2000-8. [Crossref] [PubMed]
- Tigabu BM, Davari M, Kebriaeezadeh A, et al. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review. J Crit Care 2018;48:153-9. [Crossref] [PubMed]
- Monnet X, Marik PE, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care 2016;6:111. [Crossref] [PubMed]
- Messina A, Longhini F, Coppo C, et al. Use of the Fluid Challenge in Critically Ill Adult Patients: A Systematic Review. Anesth Analg 2017;125:1532. [Crossref] [PubMed]
- Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail 2004;6:257-60. [Crossref] [PubMed]
- Vanderheyden M, Bartunek J, Goethals M. Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail 2004;6:261-8. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89. [Crossref] [PubMed]
- Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med 2004;32:660. [Crossref] [PubMed]
- Roch A, Allardet-Servent J, Michelet P, et al. NH2 terminal pro-brain natriuretic peptide plasma level as an early marker of prognosis and cardiac dysfunction in septic shock patients. Crit Care Med 2005;33:1001. [Crossref] [PubMed]
- Zhang Z, Zhang Z, Xue Y, et al. Prognostic value of B-type natriuretic peptide (BNP) and its potential role in guiding fluid therapy in critically ill septic patients. Scand J Trauma Resusc Emerg Med 2012;20:86. [Crossref] [PubMed]
- Wang F, Wu Y, Tang L, et al. Brain natriuretic peptide for prediction of mortality in patients with sepsis: a systematic review and meta-analysis. Crit Care 2012;16:R74. [Crossref] [PubMed]
- Hartemink KJ, Twisk JW, Groeneveld AB. High circulating N-terminal pro-B-type natriuretic peptide is associated with greater systolic cardiac dysfunction and nonresponsiveness to fluids in septic vs nonseptic critically ill patients. J Crit Care 2011;26:108.e1-8. [Crossref] [PubMed]
- Muller L, Louart G, Teboul JL, et al. Could B-type Natriuretic Peptide (BNP) plasma concentration be useful to predict fluid responsiveness [corrected] in critically ill patients with acute circulatory failure? Ann Fr Anesth Reanim 2009;28:531-6. [Crossref] [PubMed]
- Pirracchio R, Deye N, Lukaszewicz AC, et al. Impaired plasma B-type natriuretic peptide clearance in human septic shock. Crit Care Med 2008;36:2542. [Crossref] [PubMed]
- Sturgess DJ, Pascoe RL, Scalia G, et al. A comparison of transcutaneous Doppler corrected flow time, b-type natriuretic peptide and central venous pressure as predictors of fluid responsiveness in septic shock: a preliminary evaluation. Anaesth Intensive Care 2010;38:336. [Crossref] [PubMed]
- Mclean AS, Huang SJ, Nalos M, et al. The confounding effects of age, gender, serum creatinine, and electrolyte concentrations on plasma B-type natriuretic peptide concentrations in critically ill patients. Crit Care Med 2003;31:2611-8. [Crossref] [PubMed]
- Xu B, Yang X, Wang C, et al. Changes of central venous oxygen saturation define fluid responsiveness in patients with septic shock: A prospective observational study. J Crit Care 2017;38:13-9. [Crossref] [PubMed]
- Bone RC, Balk RA, Cerra FB, et al. Definitions for Sepsis and Organ Failure and Guidelines for the Use of Innovative Therapies in Sepsis. Chest 1992;101:1644-55. [Crossref] [PubMed]
- Asfar P, Meziani F, Hamel JF, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med 2014;370:1583-93. [Crossref]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41. [Crossref] [PubMed]
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. [Crossref] [PubMed]
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care 2011;1:1. [Crossref] [PubMed]
- Forfia PR, Watkins SP, Rame JE, et al. Relationship Between B-Type Natriuretic Peptides and Pulmonary Capillary Wedge Pressure in the Intensive Care Unit. J Am Coll Cardiol 2005;45:1667-71. [Crossref] [PubMed]
- Nikolaou NI, Goritsas C, Dede M, et al. Brain natriuretic peptide increases in septic patients without severe sepsis or shock. Eur J Intern Med 2007;18:535-41. [Crossref] [PubMed]
- Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med 2003;29:1696-702. [Crossref] [PubMed]
- Aya HD, Rhodes A, Chis SI, et al. Hemodynamic Effect of Different Doses of Fluids for a Fluid Challenge: A Quasi-Randomized Controlled Study. Crit Care Med 2017;45:e161. [Crossref] [PubMed]
- Velissaris D. High mixed venous oxygen saturation levels do not exclude fluid responsiveness in critically ill septic patients. Crit Care 2011;15:R177. [Crossref] [PubMed]
- Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008;134:172-8. [Crossref] [PubMed]
- Tomaru KK, Arai M, Yokoyama T, et al. Transcriptional activation of the BNP gene by lipopolysaccharide is mediated through GATA elements in neonatal rat cardiac myocytes. J Mol Cell Cardiol 2002;34:649-59. [Crossref] [PubMed]
- Hanford DS, Glembotski CC. Stabilization of the B-type natriuretic peptide mRNA in cardiac myocytes by alpha-adrenergic receptor activation: potential roles for protein kinase C and mitogen-activated protein kinase. Mol Endocrinol 1996;10:1719. [PubMed]
- Byung Hoon P, Moo Suk P, Young Sam K, et al. Prognostic utility of changes in N-terminal pro-brain natriuretic Peptide combined with sequential organ failure assessment scores in patients with acute lung injury/acute respiratory distress syndrome concomitant with septic shock. Shock 2011;36:109-14. [Crossref] [PubMed]
- Tung RH, Christine G, Morss AM, et al. Utility of B-type natriuretic peptide for the evaluation of intensive care unit shock. Crit Care Med 2004;32:1643-7. [Crossref] [PubMed]
- Januzzi JL, Morss A, Tung R, et al. Natriuretic peptide testing for the evaluation of critically ill patients with shock in the intensive care unit: a prospective cohort study. Crit Care 2006;10:R37. [Crossref] [PubMed]
- Monge García MI, Guijo GP, Gracia RM, et al. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med 2015;41:1247. [Crossref] [PubMed]
- Januzzi JL, Roland VK, John L, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J 2006;27:330-7. [Crossref] [PubMed]
- Heringlake M, Heide C, Bahlmann L, et al. Effects of tilting and volume loading on plasma levels and urinary excretion of relaxin, NT-pro-ANP, and NT-pro-BNP in male volunteers. J Appl Physiol (1985) 2004;97:173.
- Mcnairy M, Gardetto N, Clopton P, et al. Stability of B-type natriuretic peptide levels during exercise in patients with congestive heart failure: implications for outpatient monitoring with B-type natriuretic peptide. Am Heart J 2002;143:406-11. [Crossref] [PubMed]


