
Strategies to prevent stricture after esophageal endoscopic submucosal dissection
Introduction
Esophageal cancer, which originates in the esophageal mucosal epithelium, is the 8th most common malignancy worldwide (1). It has two main subtypes, squamous cell carcinoma, and adenocarcinoma, which account for more than 95% of malignant esophageal tumors (2). Unfortunately, malignant esophageal cancer is a deadly disease with an overall 5-year survival of less than 20%, and the highest mortality rate in East Asia (3). One positive is that the 5-year survival rate of patients can reach 95% if only the esophageal mucosal layer or superficial submucosal layer is invaded. Therefore, early diagnosis and treatment of esophageal cancer can significantly reduce the mortality and improve the prognosis of patients.
With the development of endoscopic technology, endoscopic submucosal dissection (ESD) has become the main therapeutic approach to early esophageal cancer, by which the rate of en bloc resection can reach 95% (4). However, esophageal stricture often occurs if patients suffer mucosal defects of over three-quarters of the circumference of the esophagus, and the incidence of this can range from 70% to 90%. Even worse, the incidence of postoperative stricture is almost up to 100% if patients sustain circumferential mucosal defects (5-7).
Patients with esophageal stricture mainly show different degrees of dysphagia, nausea, and vomiting, which will greatly increase the economic burden of patients and reduce their quality of life. Prevention of esophageal stricture is particularly important but there currently is no internationally consensus. This being the case, the present review aims to aggregate the prevention strategies and treatment progress in post-ESD esophageal stricture, with the aim to find better ways for preventing the occurrence of esophageal stricture after esophageal ESD.
ESD
Endoscopic resection includes endoscopic mucosal resection (EMR) and ESD, both of which are good options for treating early esophageal cancer. ESD develops on the basis of EMR, and it is more effective in the en bloc resection of an extensive lesion with an insulation-tipped (IT) knife (KD-612; Olympus Medical Systems, Tokyo, Japan) or hook knife (KD-620LR; Olympus Medical Systems), and can reduce the recurrence rate of early esophageal cancer. ESD was first introduced to the treatment of early esophageal cancer by Oyama (8). The en bloc resection rate of ESD for esophageal squamous cell carcinoma can reach 93–100%, while the complete resection rate is more than 88% in Japan (6,9).
ESD has been recommended as the first-line treatment for superficial esophageal squamous cell carcinoma by the European Society of Gastrointestinal Endoscopy (ESGE) (10). It has two popular operational approaches, namely conventional ESD and endoscopic submucosal tunnel dissection (ESTD). The main operation steps of conventional esophageal ESD involve peripheral marking, submucosal injection, circumferential mucosa incision, submucosal dissection, and wound management. However, conventional ESD is time-consuming, technical challenging, and has a high risk of adverse events such as bleeding, perforation, and stricture due to a thinner esophageal wall and narrow lumen for large lesions. On this basis, some scholars have improved conventional ESD technology and invented ESTD (11-13). Compared with conventional ESD, ESTD is an ideal method for the treatment of large esophageal lesions, as it effectively simplifies the operation steps and makes endoscopic surgery safer and faster. This is due to this method’s ability to maintain a wider view of the working field, with which efficient submucosal dissection becomes possible.
Endoscopic resection therapy is mainly used for esophageal cancer with a low risk of metastasis and complete excision. The Japanese Esophageal Society (JES) and the ESGE edited the guidelines for the diagnosis and treatment of esophageal cancer, which recommended the optimal indications for endoscopic resection of early esophageal cancer (10,14,15). In their opinion, the absolute indications for endoscopic resection were that the depth of invasion of lesions did not extend beyond the mucosal layer (T1a) and was confined within the mucosal epithelium or the lamina propria. The relative indications for endoscopic resection of esophageal cancer recommended were lesions extending up to the muscularis mucosae or slightly infiltrating the submucosa (up to 200 µm). About 50% of the lesions showed deeper (more than 200 µm) invasion into the submucosa (T1b), and in such cases, patients should be treated in the same manner as advanced carcinomas even if they are classified as superficial carcinoma.
ESD is minimally invasive, carries a low risk of serious adverse events, and is observably curative in a considerable number of patients with early esophageal cancer invading mucosa or submucosa. Furthermore, it costs less, allows quick recovery, and the long-term efficacy is comparable to that of traditional surgery (16,17). However, there are still some complications due to the influence of equipment, operators' experience, differences of lesions, and some other factors. Thus, it is necessary for doctors to pay attention to the postoperative complications of esophageal ESD, among whom esophageal stricture is one of the most common complications with the incidence of almost up to 100% in circumferential mucosal resection.
Risk factors and mechanisms of stricture after esophageal ESD
Esophageal stricture usually occurs within 2–4 weeks after ESD, which is usually accompanied by different degrees of dysphagia. Esophageal stricture can be divided into two types according to its length, shape, and lumen diameter. If the stricture is limited to a certain segment of the esophagus, and the esophageal lumen is not so obviously tortuous that the conventional gastroscope can still pass, it is called a simple stricture. If the length of the esophageal stricture exceeds 2 cm, and the esophageal lumen is obviously tortuous, or the stricture cannot be passed by conventional gastroscopy, it is called a complex stricture (18). Stricture after esophageal ESD mostly belongs to the complex refractory benign esophageal stricture (6).
The occurrence of esophageal stricture after ESD is independent of gender and age, while the size of the lesion, the depth of infiltration, the ratio of the mucosal defect of circumference, and the longitudinal length have a great impact on the stricture. And mucosal defect exceeds three-fourths of esophageal circumference or the infiltration depth of tumor exceeds lamina propria of esophageal mucosa have been demonstrated to be independent risk factors for postoperative stricture (19,20).
The mechanism of postoperative esophageal stricture has not been fully elucidated, but a large number of studies have proven that post-ESD esophageal mucosal defect is the main reason for esophageal stricture. Typically, the repair of post-ESD esophageal mucosal defect includes two forms: one is the regeneration of normal epithelial cells around the mucosal defect, and the other is granulation tissue maturing into fibrous connective tissue. In general, the two complex repair processes can be summarized as three phases: inflammatory response, epithelial proliferation, and extracellular matrix remodeling (21). Honda et al. (22) selected 6 beagle dogs who underwent esophageal EMR to establish artificial ulcer models of the esophagus, and they noticed that: ulcer formation and inflammatory cell invasion were observed in the remaining submucosa on postoperative day (POD) 2 and 4, angiogenesis and collagen fiber hyperplasia were observed after POD 7, and fibrosis of the muscularis propria was observed on POD 28. They speculated that the reason for postoperative esophageal stricture was the lowering of elasticity and movement of the esophageal wall, which was caused by the forming of fibrosis in the submucosa and muscularis propria during healing of the mucosal defect. Thus, the path to the effective prevention of esophageal stricture is clear: inhibit the inflammatory response, promote epithelial regeneration, and prevent the damage of the intrinsic muscle layer. And prevention is better started within 2–4 weeks, especially within a week, before its formation.
Prevention strategies
Its related surgical complications notwithstanding, esophageal ESD treatment for early esophageal cancer has nearly every advantage over the alternatives. Sadly, there is no internationally unified approach to the stricture. Oliveira et al. (23) found that if measures to prevent esophageal stricture were taken after esophageal ESD, the risk of esophageal stricture could be significantly reduced. Up to now, many approaches have been applied to prevent esophageal strictures such as steroids, endoscopic dilation, endoscopic stents implantation, tissue engineering, polyglycolic acid (PGA) sheets, and carboxymethyl cellulose (CMC) sheets.
Pharmacological prevention
Steroids prophylaxis
Steroids are widely used in the clinic. In particular, they can inhibit the local inflammatory response, reduce proline hydroxylase activity, and promote collagen enzyme activity to reduce fiber connective tissue formation. In some way, they can prevent the formation of a postoperative esophageal stricture (24). Up to now, the method of using steroids to prevent stricture has involved systemic steroid therapy, local injection of steroids, and steroid gel prevention.
Systemic steroid prophylaxis
Theoretically speaking, systemic steroid prophylaxis is likely to be the key measure in preventing esophageal stricture because of its powerful anti-inflammatory and anti-fibrotic effect, along with the convenience it affords to patients. However, systemic treatment with steroids, especially at high doses, is accompanied by adverse events such as immunosuppression, osteoporosis, gastrointestinal bleeding, and electrolyte imbalance, which narrows its application. In preclinical trials or clinical practice, the most common oral steroid drug is prednisolone. Generally, oral prednisolone is administered to the study group at a dose of 30 mg/day on the first few days after esophageal ESD, and then tapered gradually to 5/10 mg/day (Table 1). Perhaps this dose is the optimal administration match of oral prednisolone when considering its side effects.

Full table
A retrospective study conducted by Yamaguchi et al. (25) evaluated the efficacy of oral prednisolone for the prevention of esophageal stricture after ESD. In their study, stricture at 3 months after ESD was found in 7 of 22 patients in the preemptive endoscopic balloon dilatation (EBD) group but only in 1 of 19 patients in the oral prednisolone group. Additionally, the average number of EBD sessions required was 15.6 in the preemptive EBD group and 1.7 in the oral prednisolone group. This result clearly proves that oral prednisolone may offer a useful preventive choice for post-ESD esophageal stricture. After this study, Kataoka et al. (26) and Zhou et al. (27) dramatically demonstrated that oral prednisone therapy was safe and effective in the prevention of esophageal stricture in patients after complete or semi-circumferential ESD.
Local injection of steroid prophylaxis
Compared to systemic steroid prophylaxis, the local injection of steroids does better in reducing systemic reaction (28).
Hashimoto et al. (24) evaluated the efficacy of endoscopic triamcinolone injection (ETI) on the prevention of stricture after ESD. Patients in the study group were given triamcinolone injections equally. Finally, the proportion of patients with stricture was 19.0% in the study group and 75.0% (P<0.001) in the control group, while the number of EBD sessions was also significantly lower in the study group (mean, 1.7; range, 0–15) than in the control group (mean, 6.6; range, 0–20). Hanaoka et al. (29) obtained similar results. Wang et al. (30) conducted a meta-analysis to investigate the efficacy and safety of steroid administration on preventing esophageal stricture after circumferential ESD, and they found that steroid administration, especially local steroid injection, showed promising efficacy for stricture prevention, with a significantly reduced stricture rate and required EBD sessions during the follow-up, and local steroid injection was superior to oral steroid in EBD reduction. However, not all patients response to steroid administration. Nagami et al. (31) reported that locoregional triamcinolone injection after esophageal ESD could not reduce the risk of stricture in patients who suffered more than five-sixths circumferential esophageal mucosal defects. Hanaoka et al. (32) came to the same conclusion by analyzing the data from 127 consecutive post-ESD patients who had mucosal defects with a circumferential extent greater than three-quarters of the esophagus. They indicated that a circumferential defect extent exceeding 75% was an independent risk factor for refractory stricture despite steroid injections. In general, local injection of steroids gives patients a good alternative for preventing esophageal stricture, but there are some complications including esophageal perforation, mediastinal abscess, pleural effusion, and massive gastrointestinal bleeding (33,34). Therefore, it is necessary to have further studies on the operative details, safety, and effectiveness of this method.
New approaches to steroid prophylaxis
Numerous new approaches of steroid application have risen to relevance, such as steroid gel prophylaxis, steroid-filling prophylaxis, and intravenous injection of steroids. For instance, Mori et al. (35) found that the number (mean, 4.27; range, 0–12) of required EBD sessions in postoperative-20-day patients who received local steroid gel combined with balloon dilation was lower than in the other patients (mean, 1.6; range, 0–5) who received endoscopic multipoint steroid injection combined with balloon dilation. However, there was no significant difference in the rate of esophageal stricture between the two groups in this study. Shibagaki et al. (36) revealed that esophageal triamcinolone acetonide (TA)-filling was an effective method to prevent esophageal post-ESD stricture. In their study, the incidence of esophageal stricture of patients who received esophageal TA-filling on the 1st and 7th day after ESD was lower than average value.
Other anti-inflammatory or anti-fibrosis drug prophylaxis
Mitomycin C (MMC)
MMC prevents esophageal stricture by inhibiting fibroblast proliferation and reducing type I collagen fibers, thereby inhibiting scar formation in the process of artificial ulcer healing (37,38). Machida et al. (39) conducted a retrospective study, in which 5 patients with refractory esophageal stricture received repeated EBD after ESD, and MMC was injected into the dilated site immediately after EBD. At 4.8 months, neither recurrent dysphagia nor re-stricture appeared in any patients. Zhang et al. (40) also conducted a retrospective study on the efficacy of MMC in the treatment of esophageal stricture after ESD, which showed that MMC was effective in the treatment of esophageal stricture as well.
Botulinum toxin type A (BTX-A)
BTX-A is a kind of neurotoxin that acts on the endings of cholinergic nerves at neuromuscular junctions. It can inhibit the release of acetylcholine from the presynaptic membrane to relax smooth muscle. Some studies have shown BTX-A can also prevent scar formation after tissue injury (41). Wen et al. (42) found that the rate of esophageal stricture in the BTX-A injection group (per protocol analysis, 6.1%, 2/33; intention to treat analysis, 11.4%, 4/35) was significantly lower than that in the control group (per protocol analysis, 32.4%, 11/34; intention to treat analysis, 37.8%, 14/37), which proved endoscopic injection of BTX-A was effective in preventing post-ESD esophageal strictures.
Thymosin β4 (Tβ4)
Tβ4 is a small, abundant, naturally occurring regenerative peptide composed of 43 amino acids. It has angiogenic and anti-inflammatory activity along with the capacity to promote endothelial migration and inhibit fibrosis (43). A number of experiments and clinical trials have shown that Tβ4 can be widely applied in the healing of skin, cornea, and heart wounds, and in the attenuation of liver fibrosis (44,45). Wang et al. (46) conducted a study in 8 Bama pigs with esophageal circumferential ESD to demonstrate the safety and therapeutic effect of Tβ4 injection in preventing esophageal stricture. They found that local Tβ4 gel injection could shorten the resolution of the stricture and that this injection was associated with a lower number of EBD sessions.
In addition, some other anti-inflammatories and anti-fibrosis drugs are being tested in preclinical experiments, including N-acetylcysteine, small interfering RNA, hemostatic powder, dichroa ketone, and tranilast (47-50). Studies have confirmed their potential for the prevention of esophageal stricture, but more studies are needed to verify their effectiveness and safety.
Mechanical prevention
Endoscopic esophageal dilatation
Esophageal dilatation is a common option in post-ESD esophageal stricture prevention. It includes two main types: endoscopic bougie dilatation and EBD. In clinical practice, preventive EBD is much more common than preventive bougie dilatation. Ezoe et al. (51) found that EBD could reduce the severity of stricture and shorten the duration required for resolving the stricture. Both Yamaguchi et al. (25) and Isomoto et al. (7) also reported the effect of preventive EBD on the prevention of postoperative stricture of large-scale esophageal lesions after ESD, and the results still showed that preventive EBD had a certain effect on esophageal stricture although its efficacy was not as good as that of prophylactic oral steroid. However, endoscopic dilatation has some limitations such as requiring multiple dilatations, excessive time-consumption, risk of bleeding, perforation, bacteremia, and re-stricture.
There is currently no consensus on the start time of endoscopic dilation prevention. Most of the studies (Table 2) start within 1 week, or even immediately after ESD (5,25,50,51). Accordingly, we speculate that the best time to start preventive EBD is in the early period, i.e., within the first 2–4 weeks, especially in the first week. As collagen fiber hyperplasia was observed after a week, and fibrosis of the muscularis propria was observed after 4 weeks, as shown in animal experiments (22).

Full table
Self-help inflatable balloon
Li et al. (52,53) also reported on a novel self-help inflatable balloon to prevent esophageal stricture after circumferential ESD in 1 case and 8 cases in 2018 and 2019 respectively, which showed a high preventive effect against stricture in patients. Impressively, this novel balloon was used by the patients themselves at home and could be removed when the defects were almost healed, which made it simple, feasible, and safe for patients. Of course, the research about the novel self-help inflatable balloon was still a primary analysis, with a small sample. Nonetheless, this approach may offer a promising new option to prevent stricture.
Endoscopic stent implantation
In the past, naked metal stents were only used for esophageal stricture caused by unresectable malignant tumors, and it currently have basically been withdrawn from clinical practice because of complications such as bleeding, perforation, and immobility (54-56). Nowadays, more and more new-fashioned stents have been used to prevent esophageal stricture.
Fully covered self-expandable metal stents (SEMSs)
The advantage of fully covered SEMSs in the treatment of benign esophageal stricture is that they provide a continuous dilation effect on the narrow part of the esophagus, and can be removed at any time after the relief of esophageal stricture. Pertaining to the complex refractory benign esophageal stricture created by ESD, although the ESGE recommended against the use of SEMSs as first-line therapy, they did suggest that fully covered SEMSs be preferred over partially covered SEMSs because of their lack of embedment and ease of removability. Wen et al. (5) conducted a randomized controlled trial which demonstrated the safety and efficacy of preventive fully covered SEMSs implantation after ESD. There were also a few other studies which evaluated the safety and effectiveness of esophageal stent placement combined with the ESTD for patients with circumferential superficial esophageal lesions. Ye et al. (57) proved that esophageal stent placement with the ESTD was a safe and effective procedure in a higher en bloc resection rate with fewer or minor complications. Unfortunately, fully covered SEMS implantation has been shown to entail a few complications, such as mild chest pain, gastrointestinal bleeding, hyperplasia of granulation tissue, and stent displacement.
Biodegradable stents
Biodegradable stents can prevent not only stricture but also have any no long-term complications. A primary advantage is that the removal of stents is unnecessary which avoids the re-injury of the esophagus. At present, the poly-l-lactic acid stent has been the most widely used material, as it shows good histocompatibility, degradability, and non-toxicity.
Generally, degradation is most likely affected by pH, temperature, and body fluids. It usually occurs 11–12 weeks after implantation (58-60). The degradation products are harmless, and the stent material is partly absorbed and partly excreted through the gastrointestinal tract. Nonetheless, radiographic or endoscopic surveillance after implantation of this type of stent is recommended.
Saito et al. (60) firstly applied the poly-l-lactic acid stent to patients with stricture after esophageal ESD, and they achieved gratifying results. Pauli et al. (61) conducted a randomized controlled trial in porcine models, and they drew the conclusion that biodegradable stent placement significantly delayed the time of clinical deterioration, although this did not affect the maximum reduction in esophageal diameter or proximal esophageal dilatation. Biodegradable poly-l-lactic acid stents still have some negative aspects which include their high cost, complex implantation process, poor self-expansion, and the possibility of stent migration. Hence, randomized controlled trials with long-term follow-up are required before its widely application as a valid option to prevent stricture after esophageal ESD.
Stents covered by different substances
Recently, more and more new stents, which are covered by special material such as acellular dermal matrix (ADM), amniotic membrane, or some drugs, are being used to prevent esophageal stricture. Han et al. (62) confirmed that post-ESD esophageal stricture could be prevented by grafting an ADM sheet. Barret et al. (63) covered a plastic, non-biodegradable stent with human amniotic membrane to prove the validity in preventing esophageal stricture after ESD in pigs. Drug-eluting stents have been widely used in cardiovascular diseases such as acute coronary syndrome (64-66), and there are incremental drug-eluting stents adapted for early esophageal cancer after ESD. Anyway, more randomized clinical trials are needed to investigate the benefits and harms of stents covered by different materials.
Tissue engineering prevention
In traditional approaches, like pharmacological prevention and mechanical prevention, there exist some deficiencies in the prevention of esophageal post-ESD stricture, and so some researchers have turned their attention to new approaches. In recent years, tissue engineering technology has begun to flourish. It prevents post-ESD stricture by structurally and functionally reconstructing normal tissues and promoting early re-epithelialization after ESD.
Endoscopic injection of autologous cell suspension
Endoscopic injection of autologous cell suspension includes the operational process of harvesting cells directly from the autologous buccal cavity, skin, and adipose tissues to prepare the corresponding suspension and then to inject endoscopically. As of now, oral mucosal epithelial cells (OMECs), skin keratinocytes, and adipose tissue-derived stromal cells (ADSCs) are the main autologous cells to prevent stricture after esophageal ESD, and researchers have conducted autologous cell transplantation experiments on pigs, sheep, and dogs respectively, all of which have confirmed that endoscopic injection of autologous cell suspension has certain positive effects on the prevention of esophageal stricture after ESD. Sakurai et al. (67) showed that autologous OMEC suspension injection could prevent esophageal stricture in pigs. In their results, scar and stricture in the control lesion were found while the lesion was covered with epithelium and the luminal surface of the lesion was flat in the keratinocyte implanted lesion. Zuercher et al. (68) revealed that injecting autologous keratinocytes in the esophagus of sheep could prevent stricture formation after circumferential EMR. No esophageal stricture was observed in sheep at the 6 months follow-up, and the lumen at the injection site was flat and completely epithelialized. Honda et al. (69) found that injection of autogenous ADSC suspension could also effectively prevent esophageal stricture in the canine model. During the observation, severe stricture was found in dogs who received the only phosphate-buffered saline solution, while no stricture was found in the ADSC-injection group. Histologically, the intrinsic muscular layer of the control group was extensively destroyed, and a large amount of fibrous tissue infiltrated, while the intrinsic muscular layer of the experimental group was slightly atrophic, and fibrosis was relatively mild.
The mechanism by which autologous cell transplantation can effectively prevent esophageal stricture remains unclear. However, some scholars have speculated the reason for injecting keratinocytes to prevent esophageal stricture is that autologous cells can directly proliferate into laminated epithelium or promote the migration of peripheral epithelial cells to the defect by secreting cytokines (70). Meanwhile, the autologous cells can secrete a large number of angiogenesis-related factors, which enhance the interaction between keratinocytes and mesenchymal cells, promote the regeneration of vascularized tissues, and inhibit the fibrosis of the intrinsic muscular layer.
Autologous cell transplantation costs less and requires less time, but the limited number of isolated cells and low utilization rate are worth considering. In addition, no study on the relationship between ADSCs and esophageal tumors has been done recently. Therefore, it is not certain if injecting ADSC suspension into esophageal tumor lesions will increase the risk of tumor growth.
Cell sheet transplantation
Cell sheet transplantation involves an intermediate procedure in which cells isolated from the body are cultured in vitro, and they are transplanted into the esophageal mucosal defects with the help of certain supporting materials. Thus far, different types of cell sheets have been studied, such as autologous OMEC Sheets, autologous skin epidermal cell sheets, and autologous ADSC sheets.
The reason for cell sheets to prevent esophageal post-ESD stricture is that they provide an epithelial barrier to the lesions and secrete various growth factors and cytokines to recruit host epithelial cells to proliferate and migrate into the wound site. Cell sheets have the following advantages in preventing esophageal post-ESD stricture. First, they can attach to the defects of the esophagus, acting as soldiers to prevent the esophageal injury from being affected by food and other substances. Second, they can be easily obtained with simple operations and large samples. Third, most cell sheet transplantation belongs to autologous transplantation, and there are no transplant reactions and no severe inflammatory reactions at the wound. However, this technology entails a high cost and the process of preparation in vitro. Worse still, this method needs to requires overcome some technological problems, such as how to solve the shedding of cell sheets during esophageal peristalsis and feeding, how to prepare and preserve sheets in a sterile environment, and how to deliver them to the defect intact.
Ohki et al. (71) took the lead in combining ESD with the endoscopic transplantation of tissue-engineered cell sheets created from autologous OMECs. In their study, they found that the esophageal defect was completely cured, with no observable stricture in animals who received autologous OMEC sheet transplantation, while the control group only receiving ESD was found to have obvious fibrin mesh and host inflammation. Additionally, Kanai et al. (72) revealed that cell sheets made from autologous skin epidermal cells were as effective as OMEC sheets in preventing esophageal stricture after ESD in swine models. Soon afterward, Perrod et al. (73) reported ADSC double cell sheet transplantation could also be a viable option to prevent esophageal stricture after ESD.
Extracellular matrix stents
An extracellular matrix (ECM) stent, a kind of aussichtsreich stent, which contains a large number of active cellular ingredients and has no pro-inflammatory effect, can promote tissue repair and ulcer healing. Nieponice et al. (74) applied ECM stents to prevent esophageal stricture after circumferential EMR in dog models. Dogs treated with ECM stents in the study group had no occurrence of esophageal stricture, while dogs in the control group without ECM stent implantation had an esophageal stricture, and were accompanied with the regenerated epithelium failing to cover the mucosal defect with an inflammatory reaction. Similarly, a study was conducted in vivo by Badylak et al. (75) to prove if ECM stents colocalized with autologous muscle tissue could achieve constructive remodeling of esophageal tissue without stricture. They also confirmed that ECM stents could facilitate esophageal mucosal remodeling without stricture formation. Although some clinical trials of ECM stents have shown encouraging results, they have not yet been widely used in clinical practice.
Autologous transplantation
Transplantation of mucosa from the stomach to the esophagus
Hochberger et al. (76) reported a case of successful prevention of esophageal stricture after ESD by transplanting autologous gastric mucosa to the esophagus. They performed circumferential ESD for early squamous cell carcinoma from the hypopharynx to the cervical esophagus, as well as the second ESD in the anterior wall of the gastric sinus to gain gastric mucosa. The gastric mucosa was then transplanted to the defect of the esophagus and gently pressed against the wall by an uncovered metal stent. Twenty-four days after ESD, the esophageal mucosal defect was covered by strip gastric mucosa, and 6 months after ESD, the esophageal mucosa defect was almost completely healed. Furthermore, it was confirmed that the healing mucosa was gastric sinus mucosa, of which the Helicobacter pylori is negative. This result suggested that stomachic mucosal transplantation is effective in preventing post-ESD esophageal stricture, although clinical cases are rare.
Autologous esophageal mucosa transplantation
Chinese researchers Liao et al. (77) took the lead in carrying out autologous esophageal mucosal transplantation and achieved certain efficacy. They took in 9 patients who underwent circumferential ESD for early esophageal cancer. They then transplanted autologous esophageal mucosal patches excised by EMR by using a crescent snare from a normal esophagus site away from the lesion to the esophageal ulcer surface. During the follow-up, 8 patients did not suffer from dysphagia, while epithelialization was observed in a median time of 7.1 days, with the esophageal mucosal patch survival rate being 96.5%. However, Liu et al. (78) proposed three major questions about the technique. Regardless, these findings have expanded the directions in which research into esophageal post-ESD stricture can explore.
Autologous skin grafting
Chai et al. (79,80) were the first to perform autologous skin-grafting surgery (ASGS) to prevent esophageal stenosis after ESTD. In their study, a skin graft was harvested from the right outer thigh of the patient to sew into an oversleeve-like skin with an absorbable suture. It was then attached to a fully covered esophageal stent (FCES), and at last, the FCES was placed at the location of the artificial ulcer in the esophagus. So far, they have reported 9 patients in total (1 case in 2018 and 8 cases in 2019) who received ASGS to prevent esophageal stricture, and there was no perforation, bleeding, wound infection, or stent migration at the 7-month follow-up. Although the sample size was small, ASGS appears to be a safe and effective strategy to prevent esophageal post-ESTD stricture.
Other approaches
PGA sheets
PGA is an ideal inducer of microbial degradation and water degradation, which has been widely used in medical absorbable sutures and tissue engineering stents because of its excellent biocompatibility and nontoxicity. Studies have shown that PGA sheets are effective in preventing esophageal post-ESD stricture because they can reduce inflammatory responses and promote epithelial cell regeneration. Chai et al. (80) conducted a randomized controlled study, in which patients who underwent esophageal ESD were randomly divided into the PGA group with both PGA sheets and stent implantations and the control group with only stent implantation. They found the incidence of esophageal stricture in the PGA group was 20.5% (n=7), which was lower than that in the stent group (46.9%, n=15) (P=0.024). The frequency of EBD in the PGA group was also lower, which indicates that PGA sheets plus stents implantation are more effective in preventing post-ESD esophageal stricture compared to stent implantation alone. In addition to this, Iizuka et al. (81) and Sakaguchi et al. (82) found if PGA sheets and fibrin glue were immediately attached to the mucosal defects after ESD, the incidence of postoperative esophageal stricture and the number of EBD sessions was significantly reduced. To our excitement, studies have shown that PGA sheets combined with fibrin glue have similar efficacy in preventing post-ESD stricture compared with oral or injection of steroids.
CMC sheets
CMC sheets allow themselves to adhere to mucosal defects within minutes of exposure to moist surfaces. Usually, CMC sheets are composed of modified hyaluronic acid and CMC. Many clinical trials have shown the capability of CMC sheets in wound healing and scar inhibition (83-85). Recently, more and more studies have shown that CMC sheets can prevent esophageal stricture after ESD. Tang et al. (86) found that pigs who were immediately transplanted with CMC sheets to cover the mucosal defects had better food tolerance at the second week after ESD, and the rate of esophageal stricture in the CMC group was 71.4%, which was milder than that in the control group (100%). Using a similar experimental method, Lua et al. (87) conducted a pilot, single-center, prospective study and obtained a comparable result. It appears that the application of CMC sheets in the prevention of esophageal stricture is effective, although a randomized controlled study involving a larger number of patients and the assignment of a control group will be necessary to confirm the efficacy of CMC sheets fully.
Conclusions and outlook
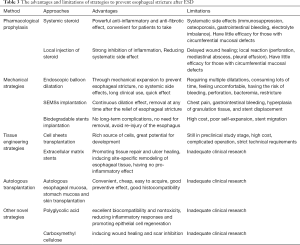
Overall, the esophageal stricture is a common post-ESD complication for which a diverse array of approaches has been investigated thoroughly to prevent, with each approach entailing its own advantages and limitations (Table 3). Among these strategies, steroid prophylaxis, particularly in its local injection application, is currently the most commonly and effectively applied strategy to prevent esophageal post-ESD stricture. In terms of endoscopic prevention strategies, this prevention measure is superior to treatment through the use of bougie dilatation, EBD, and stent implantation. Tissue engineering technology has also demonstrated the promising curative effect. Moreover, other novel strategies like esophageal mucosa transplantation, stomachic mucosa transplantation, PGA, and CMC sheets, and self-help inflatable balloon, due to their good histocompatibility and tolerance, have opened new doors in prevention of esophageal stricture. In any case, with the progress of endoscopic technology and the in-depth study of esophageal post-ESD stricture, we unhesitatingly believe that more effective and safer strategies will arrive, and the issue of esophageal post-ESD stricture will eventually be solved.
Full table
Acknowledgments
The authors appreciate the editors’ and reviewers’ valuable comments and suggestions for improving this article.
Funding: This work was supported by the Key Research Project of Hunan Province (grant number 2018SK21311).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vaghjiani RG, Molena D. Surgical management of esophageal cancer. Chin Clin Oncol 2017;6:47. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol 2015;6:20-30. [PubMed]
- Wen J, Lu Z, Yang Y, et al. Preventing stricture formation by covered esophageal stent placement after endoscopic submucosal dissection for early esophageal cancer. Dig Dis Sci 2014;59:658-63. [Crossref] [PubMed]
- Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009;70:860-6. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011;11:46. [Crossref] [PubMed]
- Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. [Crossref] [PubMed]
- Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video). Gastrointest Endosc 2010;72:255-64, 264.e1-2.
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Linghu E, Feng X, Wang X, et al. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy 2013;45:60-2. [PubMed]
- Huang R, Cai H, Zhao X, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc 2017;86:831-8. [Crossref] [PubMed]
- Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol 2016;22:435-45. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: part 2. Esophagus 2019;16:25-43.
- Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014;146:652-60.e1. [Crossref] [PubMed]
- Nakagawa K, Koike T, Iijima K, et al. Comparison of the long-term outcomes of endoscopic resection for superficial squamous cell carcinoma and adenocarcinoma of the esophagus in Japan. Am J Gastroenterol 2014;109:348-56. [Crossref] [PubMed]
- Lew RJ, Kochman ML. A review of endoscopic methods of esophageal dilation. J Clin Gastroenterol 2002;35:117-26. [Crossref] [PubMed]
- Isomoto H, Yamaguchi N, Minami H, et al. Management of complications associated with endoscopic submucosal dissection/ endoscopic mucosal resection for esophageal cancer. Dig Endosc 2013;25 Suppl 1:29-38. [Crossref] [PubMed]
- Mizuta H, Nishimori I, Kuratani Y, et al. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus 2009;22:626-31. [Crossref] [PubMed]
- Broughton G 2nd, Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg 2006;117:12S-34S. [Crossref] [PubMed]
- Honda M, Nakamura T, Hori Y, et al. Process of healing of mucosal defects in the esophagus after endoscopic mucosal resection: histological evaluation in a dog model. Endoscopy 2010;42:1092-5. [Crossref] [PubMed]
- Oliveira JF, Moura EG, Bernardo WM, et al. Prevention of esophageal stricture after endoscopic submucosal dissection: a systematic review and meta-analysis. Surg Endosc 2016;30:2779-91. [Crossref] [PubMed]
- Hashimoto S, Kobayashi M, Takeuchi M, et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011;74:1389-93. [Crossref] [PubMed]
- Yamaguchi N, Isomoto H, Nakayama T, et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011;73:1115-21. [Crossref] [PubMed]
- Kataoka M, Anzai S, Shirasaki T, et al. Efficacy of short period, low dose oral prednisolone for the prevention of stricture after circumferential endoscopic submucosal dissection (ESD) for esophageal cancer. Endosc Int Open 2015;3:E113-7. [PubMed]
- Zhou G, Yuan F, Cai J, et al. Efficacy of prednisone for prevention of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Thorac Cancer 2017;8:489-94. [Crossref] [PubMed]
- Sato H, Inoue H, Kobayashi Y, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013;78:250-7. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Takeuchi Y, et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy 2012;44:1007-11. [Crossref] [PubMed]
- Wang W, Ma Z. Steroid Administration is Effective to Prevent Strictures After Endoscopic Esophageal Submucosal Dissection: A Network Meta-Analysis. Medicine (Baltimore) 2015;94:e1664. [Crossref] [PubMed]
- Nagami Y, Ominami M, Shiba M, et al. Prediction of esophageal stricture in patients given locoregional triamcinolone injections immediately after endoscopic submucosal dissection. Dig Endosc 2018;30:198-205. [Crossref] [PubMed]
- Hanaoka N, Ishihara R, Uedo N, et al. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc Int Open 2016;4:E354-9. [Crossref] [PubMed]
- Rajan E, Gostout C, Feitoza A, et al. Widespread endoscopic mucosal resection of the esophagus with strategies for stricture prevention: a preclinical study. Endoscopy 2005;37:1111-5. [Crossref] [PubMed]
- Lee WJ, Jung HY, Kim DH, et al. Intralesional steroid injection to prevent stricture after near-circumferential endosopic submucosal dissection for superficial esophageal cancer. Clin Endosc 2013;46:643-6. [Crossref] [PubMed]
- Mori H, Rafiq K, Kobara H, et al. Steroid permeation into the artificial ulcer by combined steroid gel application and balloon dilatation: prevention of esophageal stricture. J Gastroenterol Hepatol 2013;28:999-1003. [Crossref] [PubMed]
- Shibagaki K, Ishimura N, Oshima N, et al. Esophageal triamcinolone acetonide-filling method: a novel procedure to prevent stenosis after extensive esophageal endoscopic submucosal dissection (with videos). Gastrointest Endosc 2018;87:380-9. [Crossref] [PubMed]
- Wang XQ, Liu YK, Qing C, et al. A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg 2009;63:688-92. [Crossref] [PubMed]
- Shridharani SM, Magarakis M, Manson PN, et al. The emerging role of antineoplastic agents in the treatment of keloids and hypertrophic scars: a review. Ann Plast Surg 2010;64:355-61. [Crossref] [PubMed]
- Machida H, Tominaga K, Minamino H, et al. Locoregional mitomycin C injection for esophageal stricture after endoscopic submucosal dissection. Endoscopy 2012;44:622-5. [Crossref] [PubMed]
- Zhang Y, Wang X, Liu L, et al. Intramuscular injection of mitomycin C combined with endoscopic dilation for benign esophageal strictures. J Dig Dis 2015;16:370-6. [Crossref] [PubMed]
- Xiao Z, Zhang F, Cui Z. Treatment of hypertrophic scars with intralesional botulinum toxin type A injections: a preliminary report. Aesthetic Plast Surg 2009;33:409-12. [Crossref] [PubMed]
- Wen J, Lu Z, Linghu E, et al. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointest Endosc 2016;84:606-13. [Crossref] [PubMed]
- Hannappel E. Thymosin beta4 and its posttranslational modifications. Ann N Y Acad Sci 2010;1194:27-35. [Crossref] [PubMed]
- Goldstein AL, Hannappel E, Sosne G, et al. Thymosin beta4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin Biol Ther 2012;12:37-51. [Crossref] [PubMed]
- Hong Y, Yao Q, Zheng L. Thymosin beta4 attenuates liver fibrosis via suppressing Notch signaling. Biochem Biophys Res Commun 2017;493:1396-401. [Crossref] [PubMed]
- Wang H, Shuai Q, Tang J, et al. Local Thymosin beta4 Gel Injection Prevents Esophageal Stricture after Circumferential Endoscopic Submucosal Dissection in a Porcine Model. Dig Dis 2019;37:87-92. [Crossref] [PubMed]
- Beye B, Barret M, Alatawi A, et al. Topical hemostatic powder promotes reepithelialization and reduces scar formation after extensive esophageal mucosal resection. Dis Esophagus 2016;29:520-7. [Crossref] [PubMed]
- Sato H, Sagara S, Nakajima N, et al. Prevention of esophageal stricture after endoscopic submucosal dissection using RNA-based silencing of carbohydrate sulfotransferase 15 in a porcine model. Endoscopy 2017;49:491-7. [Crossref] [PubMed]
- Wu Y, Schomisch SJ, Cipriano C, et al. Preliminary results of antiscarring therapy in the prevention of postendoscopic esophageal mucosectomy strictures. Surg Endosc 2014;28:447-55. [Crossref] [PubMed]
- Uno K, Iijima K, Koike T, et al. A pilot study of scheduled endoscopic balloon dilation with oral agent tranilast to improve the efficacy of stricture dilation after endoscopic submucosal dissection of the esophagus. J Clin Gastroenterol 2012;46:e76-82. [Crossref] [PubMed]
- Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011;45:222-7. [Crossref] [PubMed]
- Li L, Linghu E, Chai N. Using a novel self-help inflatable balloon to prevent esophageal stricture after complete circular endoscopic submucosal dissection. Dig Endosc 2018;30:790. [Crossref] [PubMed]
- Li L, Linghu E, Chai N, et al. Clinical experience of using a novel self-help inflatable balloon to prevent esophageal stricture after circumferential endoscopic submucosal dissection. Dig Endosc 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Wadhwa RP, Kozarek RA, France RE, et al. Use of self-expandable metallic stents in benign GI diseases. Gastrointest Endosc 2003;58:207-12. [Crossref] [PubMed]
- Hirdes MM, Vleggaar FP, Van der Linde K, et al. Esophageal perforation due to removal of partially covered self-expanding metal stents placed for a benign perforation or leak. Endoscopy 2011;43:156-9. [Crossref] [PubMed]
- Eloubeidi MA, Lopes TL. Novel removable internally fully covered self-expanding metal esophageal stent: feasibility, technique of removal, and tissue response in humans. Am J Gastroenterol 2009;104:1374-81. [Crossref] [PubMed]
- Ye LP, Zheng HH, Mao XL, et al. Complete circular endoscopic resection using submucosal tunnel technique combined with esophageal stent placement for circumferential superficial esophageal lesions. Surg Endosc 2016;30:1078-85. [Crossref] [PubMed]
- Walter D, van den Berg MW, Hirdes MM, et al. Dilation or biodegradable stent placement for recurrent benign esophageal strictures: a randomized controlled trial. Endoscopy 2018;50:C12. [Crossref] [PubMed]
- Imaz-Iglesia I, Garcia-Perez S, Nachtnebel A, et al. Biodegradable stents for the treatment of refractory or recurrent benign esophageal stenosis. Expert Rev Med Devices 2016;13:583-99. [Crossref] [PubMed]
- Saito Y, Tanaka T, Andoh A, et al. Novel biodegradable stents for benign esophageal strictures following endoscopic submucosal dissection. Dig Dis Sci 2008;53:330-3. [Crossref] [PubMed]
- Pauli EM, Schomisch SJ, Furlan JP, et al. Biodegradable esophageal stent placement does not prevent high-grade stricture formation after circumferential mucosal resection in a porcine model. Surg Endosc 2012;26:3500-8. [Crossref] [PubMed]
- Han Y, Guo J, Sun S, et al. Acellular dermal matrix for esophageal stricture prevention after endoscopic submucosal dissection in a porcine model. Gastrointest Endosc 2017;86:1160-7. [Crossref] [PubMed]
- Barret M, Pratico CA, Camus M, et al. Amniotic membrane grafts for the prevention of esophageal stricture after circumferential endoscopic submucosal dissection. PLoS One 2014;9:e100236. [Crossref] [PubMed]
- Feinberg J, Nielsen EE, Greenhalgh J, et al. Drug-eluting stents versus bare-metal stents for acute coronary syndrome. Cochrane Database Syst Rev 2017;8:CD012481. [PubMed]
- Giustino G, Mehran R. Drug-eluting stents and drug-eluting balloons are the best strategies to treat coronary in-stent restenosis. Evid Based Med 2016;21:90. [Crossref] [PubMed]
- Im E, Hong MK. Drug-eluting stents to prevent stent thrombosis and restenosis. Expert Rev Cardiovasc Ther 2016;14:87-104. [Crossref] [PubMed]
- Sakurai T, Miyazaki S, Miyata G, et al. Autologous buccal keratinocyte implantation for the prevention of stenosis after EMR of the esophagus. Gastrointest Endosc 2007;66:167-73. [Crossref] [PubMed]
- Zuercher BF, George M, Escher A, et al. Stricture prevention after extended circumferential endoscopic mucosal resection by injecting autologous keratinocytes in the sheep esophagus. Surg Endosc 2013;27:1022-8. [Crossref] [PubMed]
- Honda M, Hori Y, Nakada A, et al. Use of adipose tissue-derived stromal cells for prevention of esophageal stricture after circumferential EMR in a canine model. Gastrointest Endosc 2011;73:777-84. [Crossref] [PubMed]
- Horch RE, Kopp J, Kneser U, et al. Tissue engineering of cultured skin substitutes. J Cell Mol Med 2005;9:592-608. [Crossref] [PubMed]
- Ohki T, Yamato M, Murakami D, et al. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006;55:1704-10. [Crossref] [PubMed]
- Kanai N, Yamato M, Ohki T, et al. Fabricated autologous epidermal cell sheets for the prevention of esophageal stricture after circumferential ESD in a porcine model. Gastrointest Endosc 2012;76:873-81. [Crossref] [PubMed]
- Perrod G, Pidial L, Camilleri S, et al. ADSC-sheet Transplantation to Prevent Stricture after Extended Esophageal Endoscopic Submucosal Dissection. J Vis Exp 2017. [Crossref] [PubMed]
- Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc 2009;69:289-96. [Crossref] [PubMed]
- Badylak SF, Vorp DA, Spievack AR, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res 2005;128:87-97. [Crossref] [PubMed]
- Hochberger J, Koehler P, Wedi E, et al. Transplantation of mucosa from stomach to esophagus to prevent stricture after circumferential endoscopic submucosal dissection of early squamous cell. Gastroenterology 2014;146:906-9. [Crossref] [PubMed]
- Liao Z, Liao G, Yang X, et al. Transplantation of autologous esophageal mucosa to prevent stricture after circumferential endoscopic submucosal dissection of early esophageal cancer (with video). Gastrointest Endosc 2018;88:543-6. [Crossref] [PubMed]
- Liu L, Wei H, Fu J. Autologous esophageal mucosa transplantation to prevent esophageal stricture after endoscopic submucosal dissection: promising, but too early to draw a conclusion. Gastrointest Endosc 2018;88:784. [Crossref] [PubMed]
- Chai N, Zou J, Linghu E, et al. Autologous Skin-Grafting Surgery to Prevent Esophageal Stenosis After Complete Circular Endoscopic Submucosal Tunnel Dissection for Superficial Esophageal Neoplasms. Am J Gastroenterol 2019;114:822-5. [Crossref] [PubMed]
- Chai NL, Feng J, Li LS, et al. Effect of polyglycolic acid sheet plus esophageal stent placement in preventing esophageal stricture after endoscopic submucosal dissection in patients with early-stage esophageal cancer: A randomized, controlled trial. World J Gastroenterol 2018;24:1046-55. [Crossref] [PubMed]
- Iizuka T, Kikuchi D, Yamada A, et al. Polyglycolic acid sheet application to prevent esophageal stricture after endoscopic submucosal dissection for esophageal squamous cell carcinoma. Endoscopy 2015;47:341-4. [PubMed]
- Sakaguchi Y, Tsuji Y, Ono S, et al. Polyglycolic acid sheets with fibrin glue can prevent esophageal stricture after endoscopic submucosal dissection. Endoscopy 2015;47:336-40. [PubMed]
- Ten Broek RPG, Stommel MWJ, Strik C, et al. Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. Lancet 2014;383:48-59. [Crossref] [PubMed]
- Huang H, Deng M, Jin H, et al. Preventing intra-abdominal adhesions with a sodium hyaluronate carboxymethylcellulose membrane enabled visualization of hepatic microcirculation. Int J Surg 2013;11:935-43. [Crossref] [PubMed]
- Bristow RE, Montz FJ. Prevention of adhesion formation after radical oophorectomy using a sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier. Gynecol Oncol 2005;99:301-8. [Crossref] [PubMed]
- Tang J, Ye S, Ji X, et al. Deployment of carboxymethyl cellulose sheets to prevent esophageal stricture after full circumferential endoscopic submucosal dissection: A porcine model. Dig Endosc 2018;30:608-15. [Crossref] [PubMed]
- Lua GW, Tang J, Liu F, et al. Prevention of Esophageal Strictures After Endoscopic Submucosal Dissection: A Promising Therapy Using Carboxymethyl Cellulose Sheets. Dig Dis Sci 2016;61:1763-9. [Crossref] [PubMed]


